N-Rich electron acceptors: triplet harvesting in multichromophoric pyridoquinoxaline and pyridopyrazine-based organic emitters†
Abstract
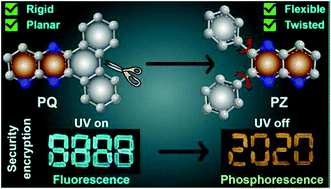
Controlling the nonradiative deactivation of triplet states and tuning the singlet–triplet energy gap are the major challenges in developing materials that exhibit thermally activated delayed fluorescence (TADF) and room temperature phosphorescence (RTP). Additionally, the conformational flexibility/rigidity around the donor–acceptor bond and the π-spacer unit significantly alters the charge transfer efficiency, subsequently impacting the relative propensity of TADF and RTP. Herein, we propose a new class of multichromophoric tridonor–acceptor (D3–A) compounds with rigid and flexible π-spacers having N-rich pyridoquinoxaline and pyridopyrazine acceptor cores, respectively. The molecule with carbazole donors meta to the quinoxaline nitrogen of the rigid pyridoquinoxaline core exhibits TADF, whereas the variation of the linkage position of carbazole to the pyridoquinoxaline as well as the twisted and flexible pyridopyrazine core shows predominantly RTP due to a relatively higher singlet–triplet energy gap. Increasing the donor strength with phenoxazine in the pyridopyrazine system leads to TADF and RTP simultaneously. Furthermore, we demonstrate the promising scope of the all-organic triplet harvesting materials in solid-state security encryption.
- This article is part of the themed collection: #RSCPoster Twitter Conference


 Please wait while we load your content...
Please wait while we load your content...