Ferroelectric low-voltage ON/OFF switching of chiral benzene-1,3,5-tricarboxamide derivative†
Abstract
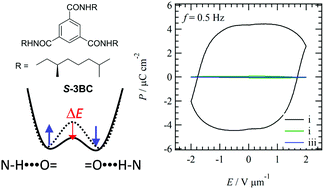
The phase transition behaviour, molecular assemblies, and dielectric responses of an N,N′,N′′-trialkylbenzene-1,3,5-tricarboxamide derivative bearing chiral (S)-3,7-dimethyloctyl chains (S-3BC) were compared with those of an N,N′,N′′-trioctadecylbenzene-1,3,5-tricarboxamide derivative (3BC) bearing achiral –CONHC14H29 chains. Chiral S-3BC showed a similar phase transition behaviour to achiral 3BC, and ferroelectric polarization–electric field (P–E) hysteresis curves were observed in the discotic hexagonal columnar (Colh) liquid crystal phase. Interestingly, the magnitudes of Pr and Et values of S-3BC were 5.5-times larger and 13-times smaller than those of 3BC because of the introduction of the chiral alkyl chains, indicating a useful method to obtain low voltage ON/OFF ratios for the switching device. Much stronger N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) hydrogen-bonding interactions were observed in S-3BC than in 3BC because of much shorter π-stacking distance and blue-shift of the intermolecular asymmetrical N–H vibrational band in the former, which effectively decreased the potential energy barrier for the dipole inversion between N–H⋯O
hydrogen-bonding interactions were observed in S-3BC than in 3BC because of much shorter π-stacking distance and blue-shift of the intermolecular asymmetrical N–H vibrational band in the former, which effectively decreased the potential energy barrier for the dipole inversion between N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) and
and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N orientations and the Et value.
O⋯H–N orientations and the Et value.


 Please wait while we load your content...
Please wait while we load your content...