Open Access Article
Open Access ArticleSmall molecule additive for low-power accumulation mode organic electrochemical transistors†
James
Nightingale
 a,
Charalampos
Pitsalidis
b,
Anna-Maria
Pappa
b,
Ellasia
Tan
a,
Katherine
Stewart
a,
Róisín M.
Owens
a,
Charalampos
Pitsalidis
b,
Anna-Maria
Pappa
b,
Ellasia
Tan
a,
Katherine
Stewart
a,
Róisín M.
Owens
 b and
Ji-Seon
Kim
b and
Ji-Seon
Kim
 *a
*a
aDepartment of Physics and Centre for Processable Electronics, Imperial College London, South Kensington Campus, London, SW7 2AZ, UK. E-mail: ji-seon.kim@imperial.ac.uk
bDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge, CB3 0AS, UK
First published on 3rd June 2020
Abstract
Low-voltage operation in accumulation mode organic electrochemical transistors (OECTs) is essential for biosensing applications and for potential use with low-voltage portable power supplies. Here, we employ a small molecule additive, dodecylbenzenesulfonate (DBSA), by adding it to the electrolyte in OECTs to improve the device performance. We find that DBSA lowers the operation voltage, increases the ON current, and increases the transconductance of the device. Such improvements are found for a range of p-type polymers including P3HT, PBTTT and DPPT-TT which have different electronic and structural properties. To investigate the device operational mechanism modulated by DBSA, we directly probe the molecular structure changes of three polymers upon charge injection (i.e. polaron formation) and correlate them to polaron density and OECT performance. We find that the electrolyte mixture (containing DBSA) enhances the electrochemical doping of the polymer by lowering the onset of oxidation and allowing the generation of a higher polaron density. For example, for P3HT the VON value decreases to 0.05 V, the ON current increases by ∼3 times, and the transconductance (gm) increases to 4 mS, which is, to the best of our knowledge, the highest transconductance of P3HT OECT reported. These results demonstrate a simple, but effective way of using a small molecule additive, such as DBSA, and a possibility to utilise otherwise unsuitable polymers with deep highest occupied molecular orbital (HOMO) levels, for low-power accumulation mode OECTs.
Introduction
Organic electrochemical devices (OEDs) utilise conducting and semiconducting polymers to engineer devices operating with high current densities and low power. OEDs have many applications which include biosensing,1–6 electrochemical energy storage,7–12 and neuromorphics.13,14 The organic electrochemical transistor (OECT) is one of the most widely deployed OEDs and consists of a channel of conjugated polymer contacted by source and drain metal electrodes.15 The polymer channel is submerged in an electrolyte and a gating electrode is exposed to the electrolyte but does not contact the channel directly.16 This allows a gate bias induced ion-redistribution which modulates the conductance of the polymer channel. The electrolyte is selected such that the polymer layer swells and allows ionic penetration into the bulk of the active channel upon application of a gate bias.17 The high concentration of ions within the polymer channel is balanced by counter charge carriers that are injected into the polymer thus causing bulk electrochemical doping/dedoping.18 This generates volumetric charging in the channel, which leads to high transconductance values (δIDS/δVDS); a figure of merit for OECTs.16The most common material used for OECTs is PEDOT:PSS, which consists of a mixture of two ionomers: negatively charged polystyrene sulphonate, PSS− blended with poly(3,4-ethylenedioxythiophene), PEDOT. PEDOT is p-type doped by the deprotonated PSS− and contains a typical hole concentration of ∼3 × 1020 holes cm−3 making it highly conductive (0.2–2100 S cm−1),19,20 thus the OECT operates in depletion mode whereby it is normally ON under no external bias application. It therefore typically requires a gate bias of approximately −0.7 V to switch it OFF, consuming power in its OFF state.15 Recently, semiconducting polymers, that are typically insulating in their pristine state, have been employed to develop accumulation mode OECT devices.16,21–23 These polymers are normally in an OFF state until electrochemically doped and switched ON thus consuming less power. Additionally, in biosensing they provide increased signal of detection in the presence of an analyte compared to depletion mode OECTs.
Semiconducting polymers swell in typical organic solvents allowing ion penetration and high transconductance.17 However, for biosensing applications it is essential to operate in aqueous environments.15,24 Due to the non-polar nature of polymers, this limits their ability to swell and thus limits performance in aqueous media. A biphasic electrolyte platform developed by Duong et al. incorporates two immiscible electrolytes, an organic layer in direct contact with the polymer and aqueous layer on top.25 The former is selected because it allows ion penetration into P3HT so it can operate as an OECT, whilst the less dense aqueous layer interfaces with the biological environment of interest. This development opens the potential to utilise a wide range of organic semiconducting polymers that have been previously developed over the past few decades for transistor and solar cell applications.
In order to implement organic semiconducting polymers in OECTs for biosensing applications, particularly with live cells or cell membranes, it is essential to operate the device at lower voltages (<1 V) in order not to adversely affect the functionality and structure of the biological systems.26–28 Low-voltage operation is also of key interest particularly important for use with low supply voltage thin-film batteries and portable devices.29 The operation voltage of the device utilising p-type doping/dedoping processes is partially governed by the highest occupied molecular orbital (HOMO) level of the polymer.25 Many polymers with deep HOMO levels are not compatible for biosensing applications (as the operation voltage would be too high when typical metal electrodes such as Au or Ag are used) despite showing promising electronic properties and high charge carrier mobility in OFET devices. In this study we report a simple but effective method for reducing the OECT operating voltage by using a small molecule additive, dodecylbenzenesulfonate (DBSA) that can be added directly to the electrolyte. Previously, DBSA has been added to PEDOT:PSS formulations to act as a surfactant to improve thin film processing.30 Herein, we demonstrate how DBSA can effectively reduce operating-voltages, enhance performance via increasing the ON current and transconductance, and lower the power consumption of the OECT device when added to the electrolyte. By using a range of p-type polymers, we confirm that this method can be utilised in organic semiconducting polymers with deep HOMO levels for high performance OECTs with operating voltages low enough for biosensing applications.
We first demonstrate the benefit of adding DBSA (Fig. 1) to the electrolyte (tetrabutylammonium hexafluorophosphate, TBA PF6) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to enhance OECT performance consisting of P3HT polymer channels. We reveal that adding DBSA increases the ON current and transconductance and decreases the operation voltage resulting in significant enhancement in device performance. We demonstrate this further by applying the DBSA additive to OECTs fabricated with other conjugated polymers including DPPT-TT and PBTTT. We select PBTTT because it has been shown to be a high performance material for use in OECTs.25 DPPT-TT, on the other hand, has yet to be reported in the literature for use in OECTs, which led us to investigate whether its well-known high performance in OFETs translates to high performance in OECTs.30–32 We observe the same effects of adding DBSA to the gating electrolyte for both DPPT-TT and PBTTT as for P3HT.
1 ratio to enhance OECT performance consisting of P3HT polymer channels. We reveal that adding DBSA increases the ON current and transconductance and decreases the operation voltage resulting in significant enhancement in device performance. We demonstrate this further by applying the DBSA additive to OECTs fabricated with other conjugated polymers including DPPT-TT and PBTTT. We select PBTTT because it has been shown to be a high performance material for use in OECTs.25 DPPT-TT, on the other hand, has yet to be reported in the literature for use in OECTs, which led us to investigate whether its well-known high performance in OFETs translates to high performance in OECTs.30–32 We observe the same effects of adding DBSA to the gating electrolyte for both DPPT-TT and PBTTT as for P3HT.
To elucidate the effects of DBSA and the electrolyte mixture (containing DBSA), we use electrochemical resonant Raman spectroscopy (ERRS), which provides a powerful tool to probe changes to the vibrational modes during polaron (charge) formation in polymers. Polarons are formed in conjugated polymers when the polymer becomes charged. Owing to the strong electron–phonon coupling in conjugated polymers, the charge is accompanied by a distortion of the equilibrium position of the local nuclei resulting in changes to the vibrational modes (so called lattice relaxation). ERRS generates polarons in the polymer via the application of an oxidising potential (electrochemically doping) in a three-electrode electrochemical cell, whilst probing changes of the vibrational modes of the polymer in situ using resonant Raman spectroscopy.33
We find that the addition of DBSA acts to generate polarons at a lower potential and to a higher density. Ultimately this decreases the operating-voltage of the OECT and paves the way to implementing other high-performance p-type polymers with deeper HOMO levels as channel materials for OECTs for a variety of applications, including biosensing.
Experimental
Materials
Details of the polymers used in this work are as follows: RR-P3HT (95.2% regioregularity) was bought from Merck, with weight-average molecular weight (Mw) ∼36.6 kg mol−1 and polydispersity index of 2; PBTTT-C14 was purchased from Sigma Aldrich, with weight-average molecular weight (Mw) 12 kDa; DPPT-TT-C10C8 was purchased from 1-material (1M) with weight-average molecular weight (Mw) 87.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The PDMS well contained the electrolyte with a total volume of 300 μl. TBA PF6 electrolytes and TBA PF6
1 ratio. The PDMS well contained the electrolyte with a total volume of 300 μl. TBA PF6 electrolytes and TBA PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBSA mixed electrolytes (in both ACN and DCM) had a concentration of 0.1 M. The ratio of TBA PF6
DBSA mixed electrolytes (in both ACN and DCM) had a concentration of 0.1 M. The ratio of TBA PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBSA was kept constant at 1
DBSA was kept constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for all mixed electrolyte measurements.
1 for all mixed electrolyte measurements.
Results and discussion
First, we demonstrate the effect of DBSA as a small molecule electrolyte additive, which enhances the electrochemical doping mechanism and device characteristics of OECTs fabricated from conjugated polymers. We start with P3HT and extend the application to PBTTT and DPPT-TT.
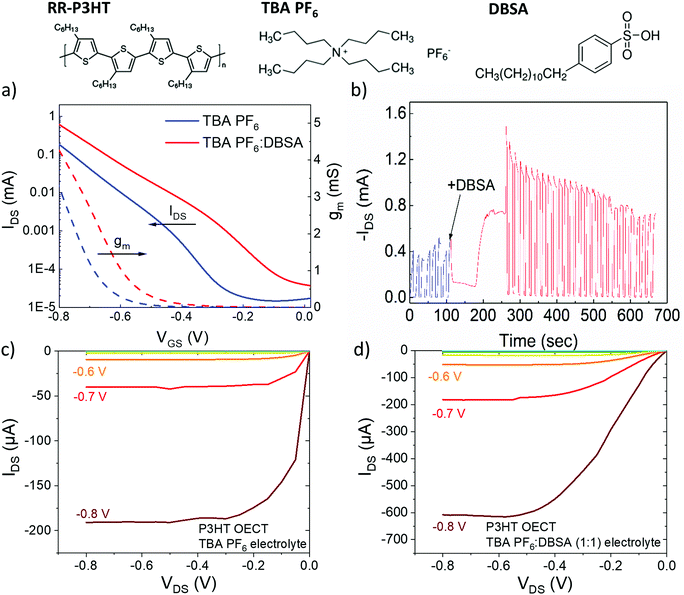
Fig. 1 shows the transfer characteristics of OECTs fabricated with P3HT as a channel material and TBA PF6 (blue) and TBA PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBSA (1
DBSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (red) in acetonitrile (ACN) as a gating electrolyte. With TBA PF6 the ON currents of the device are 0.04 mA at a gate bias of −0.7 V. The ON/OFF ratio is ∼104 with an OFF current ∼2 × 10−5 mA. When DBSA is added to the TBA PF6 gating electrolyte the ON current increases by over 4 times to 0.18 mA with no significant change in the ON/OFF ratio (∼104) and an OFF current of ∼4 × 10−5 mA. The maximum (measured) transconductance, gm (at VGS = −0.8 V), increases from 3 mS (no DBSA) to 4 mS (with DBSA). The sub VON characteristics with both electrolytes are flat and show sharp turn-on. Most notably DBSA causes a significant decrease in the operation voltage with a drop in VON value from ∼0.70 V to ∼0.58 V (ΔVON = 0.12 V) (Fig. S1 for IDS1/2 plots, ESI†). Fig. 1b shows the transient gate bias pulse measurements (VGS = −0.6 V, VDS = −0.6 V). During a gate bias of −0.6 V (in TBA PF6 electrolyte) there is a drain current of ∼0.4 mA. Adding DBSA causes a large amplification of the IDS by ∼3 × (from 0.4 mA to 1.2 mA), whilst the transient turn-on/turn-off characteristics remain fast. Adding DBSA also has a positive effect on the output characteristics of P3HT (Fig. 1c and d): IDS increases ∼3 times from ∼−190 μA without DBSA to ∼−600 mA with DBSA (VGS = −0.8 V).
1) (red) in acetonitrile (ACN) as a gating electrolyte. With TBA PF6 the ON currents of the device are 0.04 mA at a gate bias of −0.7 V. The ON/OFF ratio is ∼104 with an OFF current ∼2 × 10−5 mA. When DBSA is added to the TBA PF6 gating electrolyte the ON current increases by over 4 times to 0.18 mA with no significant change in the ON/OFF ratio (∼104) and an OFF current of ∼4 × 10−5 mA. The maximum (measured) transconductance, gm (at VGS = −0.8 V), increases from 3 mS (no DBSA) to 4 mS (with DBSA). The sub VON characteristics with both electrolytes are flat and show sharp turn-on. Most notably DBSA causes a significant decrease in the operation voltage with a drop in VON value from ∼0.70 V to ∼0.58 V (ΔVON = 0.12 V) (Fig. S1 for IDS1/2 plots, ESI†). Fig. 1b shows the transient gate bias pulse measurements (VGS = −0.6 V, VDS = −0.6 V). During a gate bias of −0.6 V (in TBA PF6 electrolyte) there is a drain current of ∼0.4 mA. Adding DBSA causes a large amplification of the IDS by ∼3 × (from 0.4 mA to 1.2 mA), whilst the transient turn-on/turn-off characteristics remain fast. Adding DBSA also has a positive effect on the output characteristics of P3HT (Fig. 1c and d): IDS increases ∼3 times from ∼−190 μA without DBSA to ∼−600 mA with DBSA (VGS = −0.8 V).
The state-of-the art P3HT OECT device performance reported so far achieves an ON current of 0.3 mA, VON of −0.2 V, and a transconductance of 2 mS.23 We show that the VON value can be decreased to 0.05 V, the ON current increased by ∼3 times, and the transconductance, gm increased from 3 mS to 4 mS. To the best of our knowledge this is the highest transconductance of a P3HT OECT reported, with the next highest being 2 mS.23
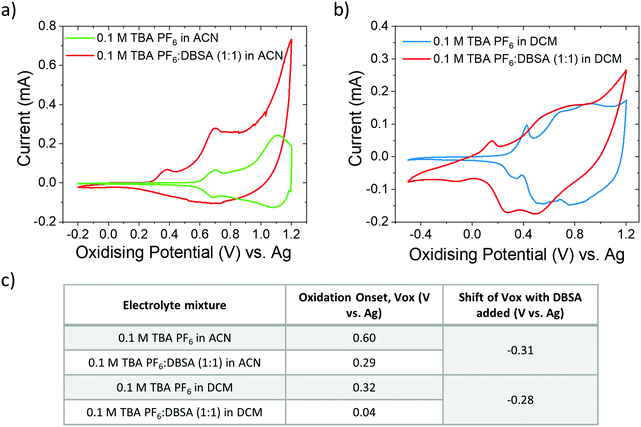
The addition of DBSA appears to improve device characteristics by lowering the operation voltage and increasing the drain current, thus lowering the power consumption of the device. This indicates the ability of DBSA to improve the ion-induced modulation of the polymer channel conductance. This correlates with a decrease in the oxidation onset (from 0.60 V to 0.29 V vs. Ag) of P3HT thin films when DBSA is present in the electrolyte mixture (CV Fig. 2). The two oxidation peaks of P3HT in TBA PF6 electrolyte (green curve) (Fig. 2a) have previously been assigned to the oxidation of crystalline (0.6–0.8 V) and amorphous (1.0–1.2 V) phases.33,35,36 The relative ratio amplitude of the crystalline and amorphous oxidation peaks (Icrystalline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Iamorphous) is 0.27
Iamorphous) is 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in TBA PF6 electrolyte and remains approximately the same when DBSA is added (0.26
1 in TBA PF6 electrolyte and remains approximately the same when DBSA is added (0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This indicates that both crystalline and amorphous polymer phases sufficiently swell and allow ion penetration to an equal extent in both electrolytes suggesting that DBSA does not alter the interactions between the solvent and different polymer phases.
1). This indicates that both crystalline and amorphous polymer phases sufficiently swell and allow ion penetration to an equal extent in both electrolytes suggesting that DBSA does not alter the interactions between the solvent and different polymer phases.
It is common to use dichloromethane (DCM) as an electrolyte solvent for biosensing applications.23 Similarly to ACN, DCM can sufficiently dissolve salts, and it can efficiently swell polymer films without dissolving individual chains. DCM, however, is far less polar than ACN and is therefore largely immiscible with water facilitating an interface with biological environments and media.25
Interestingly, the oxidation onset (using TBA PF6 salt only) occurs at a lower potential in DCM compared to ACN. This is due to the higher polarity of ACN which may have two possible effects leading to different oxidation onsets measured for the polymer. Firstly, there is a higher relative energy distance (RED) between P3HT and ACN compared to P3HT and DCM, which makes DCM a better solvent causing the polymer film to swell more (without fully dissolving the film). Greater swelling of the polymer is expected to reduce resistance to ion penetration. This has been previously calculated for PBTTT using the Hansen solubility parameter space where the RED for DCM is <1 (0.64) and ACN >1 (2.6).25,37 We calculate the RED between P3HT and DCM (1.01) and between P3HT and ACN (2.76) using solubility parameters reported by Zhang et al.38 Secondly, the higher polarity of ACN will more strongly solvate the electrolyte ions, which can cause an overpotential in the electrochemical oxidation of the polymer film.22 The primary effect of DBSA in both solvents is a significant reduction in the oxidation onset of the polymer film.
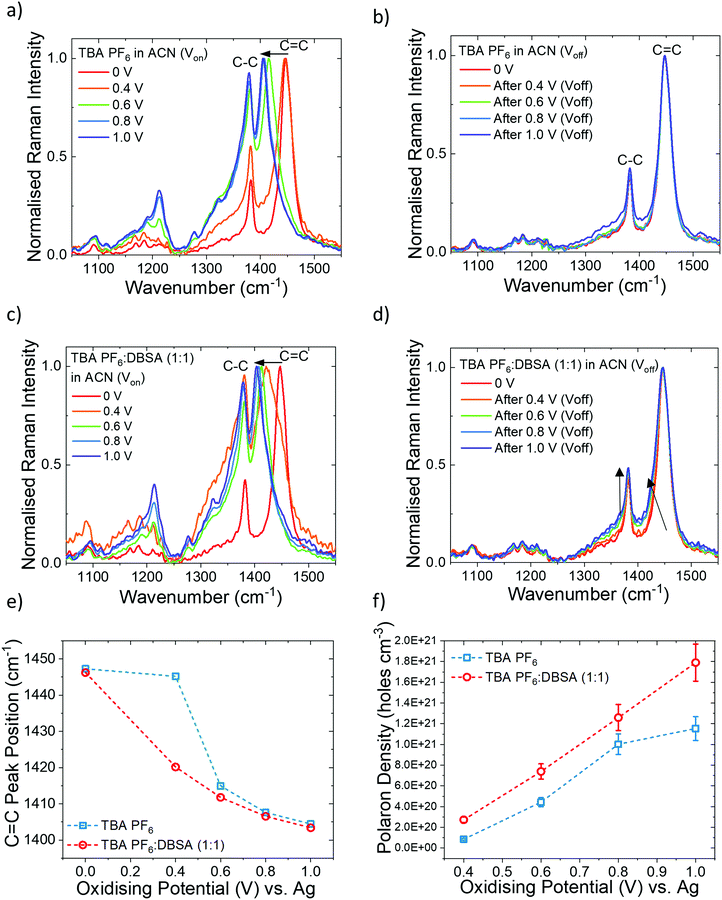
To elucidate the doping mechanism of the admixture of DBSA and TBA PF6 electrolyte we employ ERRS to directly probe polaron formation and associated conformation changes of the polymer in situ. Using ERRS we can distinguish between primary and secondary doping effects of DBSA. Fig. 3a shows the ERRS spectra of P3HT in TBA PF6 electrolyte during a series of oxidising potential applications. The most intense peak (1448 cm−1) is assigned to the intra-ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond vibrational mode and the weaker peak (1382 cm−1) is assigned to the intra-ring C–C bond vibrational mode.39 The ERRS spectra as a function of applied oxidising potential displays the characteristic Raman signatures of hole polarons forming in different polymer phases; holes form first in the crystalline phase at lower potentials (∼0.6 V) followed by the amorphous phase at higher potentials (∼1.0 V). These spectral changes we observe during Vox provide information on conformational changes of the P3HT during polaron formation.33 Most notably is the downshift of the C
C bond vibrational mode and the weaker peak (1382 cm−1) is assigned to the intra-ring C–C bond vibrational mode.39 The ERRS spectra as a function of applied oxidising potential displays the characteristic Raman signatures of hole polarons forming in different polymer phases; holes form first in the crystalline phase at lower potentials (∼0.6 V) followed by the amorphous phase at higher potentials (∼1.0 V). These spectral changes we observe during Vox provide information on conformational changes of the P3HT during polaron formation.33 Most notably is the downshift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak to lower wavenumber (from 1448 to 1404 cm−1) and an increase in the relative intensity of the C–C/C
C peak to lower wavenumber (from 1448 to 1404 cm−1) and an increase in the relative intensity of the C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks (from 0.38 to 0.93), indicating that the C
C peaks (from 0.38 to 0.93), indicating that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond force constant weakens and the polymer adopts a more planar conformation during polaron formation. After discharging the film (0 V) to its neutral state, we observe the Raman spectra revert back to its original shape and position (Fig. 3b), which indicates the conformational reorganisation during polaron formation is entirely reversible.
C bond force constant weakens and the polymer adopts a more planar conformation during polaron formation. After discharging the film (0 V) to its neutral state, we observe the Raman spectra revert back to its original shape and position (Fig. 3b), which indicates the conformational reorganisation during polaron formation is entirely reversible.
When a low concentration of DBSA (0.05 M) is added to the electrolyte mixture (TBA PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBSA (1
DBSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) similar spectral changes occur during polaron formation (Fig. 3c). Monitoring the C
1)) similar spectral changes occur during polaron formation (Fig. 3c). Monitoring the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak position as a function of oxidising potential (Fig. 3d) reveals that the conversion of neutral polymer to charged polymer has already occurred at 0.4 V when DBSA is present (red line) compared to 0.6 V when DBSA is absent (blue line). The formation of hole polarons at a lower oxidising potential correlates with the lower operation voltage in the OECT when DBSA is added to the gating electrolyte. Furthermore, when DBSA is added to the electrolyte we measure a higher polaron density generated in the film (1.79 × 1021 holes cm−3 at 1.0 V) compared to just TBA PF6 (1.16 × 1021 holes cm−3 at 1.0 V) (Fig. 3e). The method used for calculating polaron density has been previously reported.33 The polaron density generated in this study is typical of the polaron density generated in OECT devices.22 This correlates well with the higher drain current measured in the OECT device when DBSA is added. Thus, we confirm that DBSA enhances the ion-induced modulation of the device by altering the primary electrochemical doping of the polymer.
C peak position as a function of oxidising potential (Fig. 3d) reveals that the conversion of neutral polymer to charged polymer has already occurred at 0.4 V when DBSA is present (red line) compared to 0.6 V when DBSA is absent (blue line). The formation of hole polarons at a lower oxidising potential correlates with the lower operation voltage in the OECT when DBSA is added to the gating electrolyte. Furthermore, when DBSA is added to the electrolyte we measure a higher polaron density generated in the film (1.79 × 1021 holes cm−3 at 1.0 V) compared to just TBA PF6 (1.16 × 1021 holes cm−3 at 1.0 V) (Fig. 3e). The method used for calculating polaron density has been previously reported.33 The polaron density generated in this study is typical of the polaron density generated in OECT devices.22 This correlates well with the higher drain current measured in the OECT device when DBSA is added. Thus, we confirm that DBSA enhances the ion-induced modulation of the device by altering the primary electrochemical doping of the polymer.
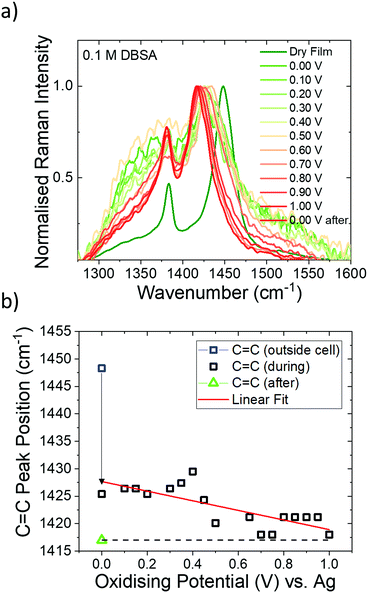
We are able to clarify that the electrochemical doping of the polymer is still predominantly caused by the formation of ion pairs between charged polymer and PF6− anions, as DBSA itself causes irreversible doping of the polymer (Fig. 4). Fig. 4a shows the behaviour of DBSA in an electrochemical cell with no other salts present by exposing a P3HT thin film to 0.1 M DBSA in ACN electrolyte and measuring in situ ERRS. We observe the typical hole polaron signature in P3HT even at 0 V, indicating the ability of DBSA to chemically dope the polymer film when no other salts are present. We monitor the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak position as a function of oxidising potential and find the doping level has a minor dependence on applied bias indicated by the C
C peak position as a function of oxidising potential and find the doping level has a minor dependence on applied bias indicated by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak downshifting from 1425 cm−1 to 1417 cm−1 (Fig. 4b); the electric field causes more DBSA anions (dissociated from protons) to drift and penetrate into the P3HT film causing further doping. After discharge (0 V) the film is irreversibly doped and the C
C peak downshifting from 1425 cm−1 to 1417 cm−1 (Fig. 4b); the electric field causes more DBSA anions (dissociated from protons) to drift and penetrate into the P3HT film causing further doping. After discharge (0 V) the film is irreversibly doped and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak position remains at 1417 cm−1 (green triangle Fig. 4a). Further evidence for irreversible p-type doping caused by DBSA is provided by changes in the UV-Vis absorbance spectra, HOMO level, and work function (see Fig. S2, ESI†). Thus, we elucidate that the mixed ionic electrolyte (with DBSA and TBA:PF6) causes reversible electrochemical doping of the polymer caused by the formation of ion pairs between charged polymer and PF6− anions. This suggests that the effect of DBSA in the mixed electrolyte is to modify the interaction between the polymer and the electrolyte allowing electrochemical doping at a lower voltage. DBSA has previously been used as a surfactant added to PEDOT:PSS formulations to improve wettability.30 We believe that DBSA could be having a similar effect on P3HT, interacting with the polymer and acting as a surfactant to enhance the strength of the interactions between the polymer and the electrolyte. In other words, DBSA may modulate the RED between the polymer and the solvent. We demonstrated the effect of RED between the solvent and the polymer on oxidation onset during cyclic voltammetry measurements: there is a lower RED between DCM and P3HT compared to ACN and P3HT, which results in a reduction in the oxidation onset of 0.28 V (Fig. 2). Thus, the RED between the polymer and the electrolyte solvent appears to influence the injection barrier into the film. By adding DBSA to the electrolyte, it may reduce the RED, thus causing a higher mass in the polymer film (solvent and ions), ultimately decreasing the injection barrier into the film.
C peak position remains at 1417 cm−1 (green triangle Fig. 4a). Further evidence for irreversible p-type doping caused by DBSA is provided by changes in the UV-Vis absorbance spectra, HOMO level, and work function (see Fig. S2, ESI†). Thus, we elucidate that the mixed ionic electrolyte (with DBSA and TBA:PF6) causes reversible electrochemical doping of the polymer caused by the formation of ion pairs between charged polymer and PF6− anions. This suggests that the effect of DBSA in the mixed electrolyte is to modify the interaction between the polymer and the electrolyte allowing electrochemical doping at a lower voltage. DBSA has previously been used as a surfactant added to PEDOT:PSS formulations to improve wettability.30 We believe that DBSA could be having a similar effect on P3HT, interacting with the polymer and acting as a surfactant to enhance the strength of the interactions between the polymer and the electrolyte. In other words, DBSA may modulate the RED between the polymer and the solvent. We demonstrated the effect of RED between the solvent and the polymer on oxidation onset during cyclic voltammetry measurements: there is a lower RED between DCM and P3HT compared to ACN and P3HT, which results in a reduction in the oxidation onset of 0.28 V (Fig. 2). Thus, the RED between the polymer and the electrolyte solvent appears to influence the injection barrier into the film. By adding DBSA to the electrolyte, it may reduce the RED, thus causing a higher mass in the polymer film (solvent and ions), ultimately decreasing the injection barrier into the film.
Lastly, we note that the ionic strength of the mixed DBSA:TBA PF6 electrolyte is the same as the neat TBA PF6 electrolyte. Ionic strength of a solution, I, is dependent on the concentration of ions in the solution: I = 1/2Σcizi2, where ci is the molar concentration of all ions in the solution, and zi is the charge number of the ion. Since we control the molar concentration to be the same for both the mixed and neat electrolyte (0.1 M for both), and the charge number of all ions is parity (TBA+vs. H+ and PF6−vs. DSBA−), we are thus able to rule out the possibility of ionic strength causing the effect of DBSA observed in lowering the oxidation onset of the polymer thin films.
After discharging the film (0 V) exposed to the mixed electrolyte (TBA PF6:DBSA) the Raman signatures revert to that of the original, neutral polymer, revealing no change in polymer conformation or molecular morphology, and thus ruling out any secondary doping effect of DBSA (Fig. 3d). There is, however, a low intensity polaron signature evident from the slight broadening of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak to lower wavenumber and a slight increase in C–C relative peak intensity (indicated by arrows in Fig. 3d). This reveals that the doping process isn’t completely reversible (at 0.6 V and above) and leads to a low density of polarons remaining in the film. This correlates with the very low level drain current during no gate bias (0 V) in the transient gate bias pulse measurements (Fig. 1b), which wasn’t observed before DBSA was added.
C peak to lower wavenumber and a slight increase in C–C relative peak intensity (indicated by arrows in Fig. 3d). This reveals that the doping process isn’t completely reversible (at 0.6 V and above) and leads to a low density of polarons remaining in the film. This correlates with the very low level drain current during no gate bias (0 V) in the transient gate bias pulse measurements (Fig. 1b), which wasn’t observed before DBSA was added.
Currently there is much debate within the field of organic mixed ionic-electronic devices about the extent of bulk doping (volumetric effect) of organic conjugated polymers with alkyl side chains using common organic electrolytes. Using ERRS we confirm the volumetric effect in polymer thin films (Fig. S3, ESI†). We compare the strength of the Raman signals from films of different thickness (124 nm vs. 270 nm) with controlled laser focus (micron-size depth resolution) for each measurement. We observe a signal twice as strong for the thicker film (240 nm), which is just over twice the thickness of the 124 nm film. The signal strength is governed by the Raman scattering cross section, which in turn is dependent upon the number of Raman active species present.39 785 nm excitation is resonant with the polaron absorption and thus a higher intensity indicates a greater number of polarons generated in the film (due to its greater thickness and bulk doping effect). This is in close agreement with our measurement of the number of charges extracted after the oxidising pulse for which twice as many were extracted from the thicker film (4.7 × 1016vs. 2.3 × 1016 holes). Our results support the notion that bulk electrochemical doping occurs in organic conjugated polymers even with alkyl side chains using acetonitrile-based electrolytes.
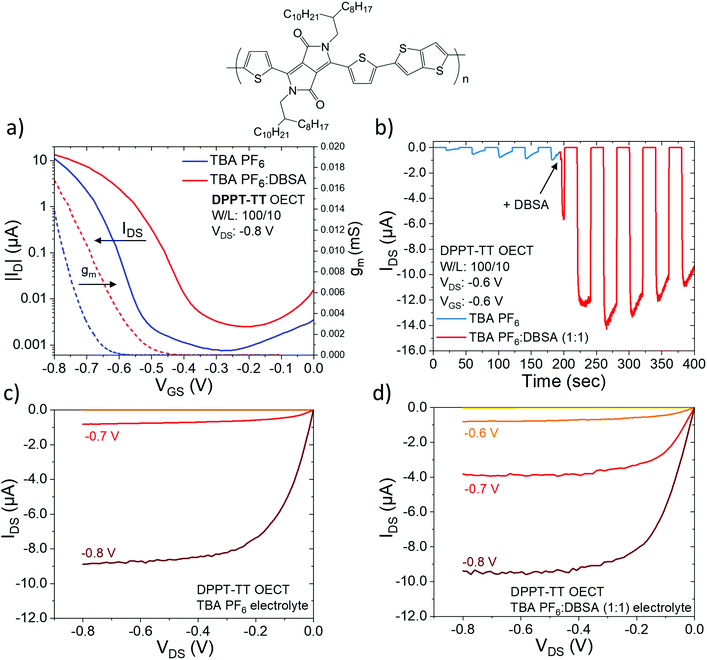
Now we show the beneficial effects of DBSA addition to other conjugated polymer OECTs including DPPT-TT and PBTTT devices, which suggests its effect can be extended and applied to other conjugated polymer systems. Fig. 5a shows the transfer curve characteristics of OECTs fabricated with DPPT-TT and gating electrolytes comprising TBA PF6 (black) and TBA PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBSA (1
DBSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (red) in ACN. With the TBA PF6 electrolyte the ON current of the device is ∼−1.0 μA at a gate bias of −0.7 V. The ON/OFF ratio is ∼104 with an OFF current of ∼−10−4 μA. When DBSA is added to the gating electrolyte the ON current increases to ∼−10.0 μA, and whilst the OFF current also increases the ON/OFF ratio remains ∼104. Most remarkable is the significant decrease in the operation voltage upon addition of DBSA with a drop in VON value from ∼0.63 V to ∼0.48 V (ΔVON = −0.15 V) (Fig. S1 for IDS1/2 plots, ESI†). Fig. 5b shows the transient gate bias pulse measurements. During a gate bias of −0.8 V (in TBA PF6 electrolyte) there is a low drain current of ∼−0.8 μA. Adding DBSA causes a large amplification of the IDS by ∼18 × (from 0.8 μA to 14.0 μA), whilst the transient turn-on/turn-off characteristics remain fast. Albeit to a lesser extent the output characteristics are also improved upon DBSA addition: IDS increases ∼4 times from −1 μA to −4 μA (at VGS = 0.7 V) (Fig. 5c and d).
1) (red) in ACN. With the TBA PF6 electrolyte the ON current of the device is ∼−1.0 μA at a gate bias of −0.7 V. The ON/OFF ratio is ∼104 with an OFF current of ∼−10−4 μA. When DBSA is added to the gating electrolyte the ON current increases to ∼−10.0 μA, and whilst the OFF current also increases the ON/OFF ratio remains ∼104. Most remarkable is the significant decrease in the operation voltage upon addition of DBSA with a drop in VON value from ∼0.63 V to ∼0.48 V (ΔVON = −0.15 V) (Fig. S1 for IDS1/2 plots, ESI†). Fig. 5b shows the transient gate bias pulse measurements. During a gate bias of −0.8 V (in TBA PF6 electrolyte) there is a low drain current of ∼−0.8 μA. Adding DBSA causes a large amplification of the IDS by ∼18 × (from 0.8 μA to 14.0 μA), whilst the transient turn-on/turn-off characteristics remain fast. Albeit to a lesser extent the output characteristics are also improved upon DBSA addition: IDS increases ∼4 times from −1 μA to −4 μA (at VGS = 0.7 V) (Fig. 5c and d).
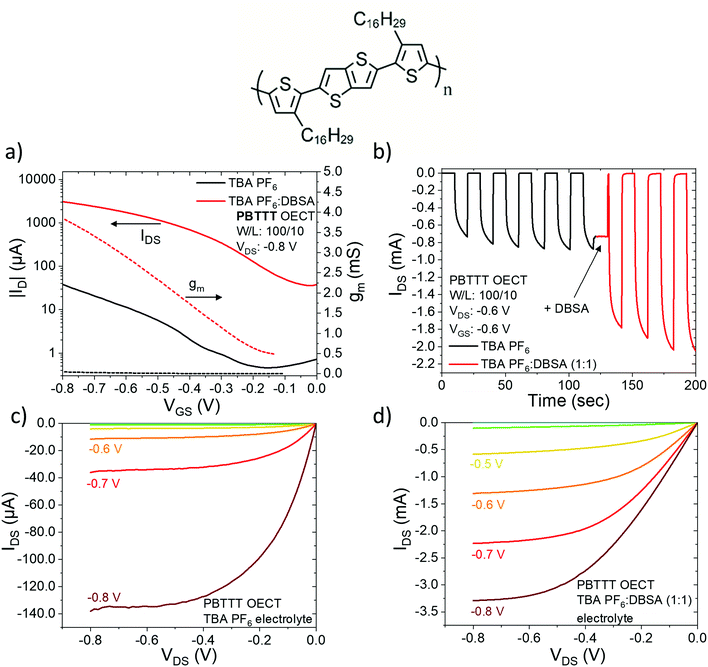
We report, for the first time, that DPPT-TT is a lower performance channel material compared to P3HT and PBTTT. For DPPT-TT, the ON current (∼−1.0 μA), VON value (∼0.63 V), and transconductance (0.012 mS), are far below the performance of P3HT and PBTTT. Still, the addition of DBSA to the electrolyte is effective in enhancing its performance and results in a significant increase in the ON current and decrease in the operation voltage. The positive effect of adding DBSA to the electrolyte extends not only to DPPT-TT but also PBTTT, where it appears to have an even stronger effect.
Fig. 6a shows the transfer characteristics of OECTs fabricated with PBTTT with the two different electrolytes. Without DBSA the ON current of the device is ∼−35 μA at a gate bias of −0.8 V and the ON/OFF ratio is ∼102 with an OFF current ∼10−1 μA. This is of relatively poor performance, but when DBSA is added to the gating electrolyte it has a huge effect on the ON current which increases to −3.1 mA with no change in the ON/OFF ratio. The transconductance, gm increases from 0.05 mS (no DBSA) to 3.9 mS (with DBSA). Again, there is a significant decrease in the operation voltage with a drop in the VON value from 0.35 V to 0.12 V (ΔVON = 0.23 V) (Fig. S1 for IDS1/2 plots, ESI†). Adding DBSA also has a strong and positive effect on the transient gate bias pulse characteristics (Fig. 6b) as well as the output characteristics of PBTTT (Fig. 6c and d): IDS increases ∼25 times from ∼−140 μA without DBSA to ∼−3.3 mA with DBSA.
The highest performing PBTTT OECT device reported displays a transconductance ≈ 5 mS, VON = −0.35 V, and an ON current of ∼0.15–0.20 mA.25 Although the device performance we report here without DBSA is relatively poor compared to the state-of-the-art, we manage to increase the transconductance of PBTTT to 3.9 mS, and achieve the highest drain current (0.31 mA at V = −0.8 V) and lowest VON value (0.12 V) of any PBTTT device previously reported. This demonstrates the significance of DBSA in enhancing OECT device performance.
In summary, we demonstrate the benefit of the gate electrolyte mixture (TBA PF6:DBSA) which improves device performance of OECTs by increasing the drain current and reducing the operation voltage. This is applicable to various conjugated polymers including P3HT, PBTTT, and DPPT-TT. Importantly, we unveil the operational mechanism of these conjugated polymer based OECT devices with mixed ion gating electrolyte showing a strong gate-modulated p-type doping/dedoping of the polymer.
Conclusions
We present a small molecule additive, DBSA, that can be added to the gating electrolyte of accumulation mode OECTs resulting in lower operation voltage and higher ON currents, thus decreasing power consumption. We directly probe polaron formation in P3HT via electrochemical doping to elucidate that the mixed TBA PF6:DBSA electrolyte generates polarons by the same mechanism (reversible electrochemical doping) as common electrolyte salts (e.g., TBA PF6). Importantly, polarons are generated at a lower potential and to a higher density. We confirm that no secondary doping effects on the polymer conformation or morphology take place. We apply the small molecule additive to other conjugated polymers, PBTTT and DPPT-TT for accumulation mode OECT operation. We observe the same effect and achieve an increase in the transconductance of PBTTT from 0.05 mS to 3.9 mS upon addition of DBSA. Importantly, we observe a decrease in VON values for all polymers (PBTTT: 0.23 V, P3HT: 0.12 V, DPPT-TT: 0.15 V). Decreasing the operating-voltage of accumulation-mode OECTs, enables the implementation of higher performance p-type polymers with deeper HOMO levels as channel materials for OECTs, extending the repertoire of applications including biosensing. Interestingly, we find the behaviour of DBSA heavily dependent on the presence of other electrolyte salts. When alone, DBSA acts as an irreversible chemical dopant. When mixed with other salts however, (e.g. TBA PF6) it enhances the reversible electrochemical doping properties of the electrolyte. Thus, this small molecule additive can be used to dope the electrolyte and enhance device performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the UK EPSRC for studentships under DTG and the Plastic Electronics Centre for Doctoral Training (EP/L016702/1) and the Imperial College High Performance Computing Service for DFT calculations. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 723951). The author (E. Tan) thanks the National Physical Laboratory (NPL) for a CASE studentship.Notes and references
- A. Giovannitti, K. J. Thorley, C. B. Nielsen, J. Li, M. J. Donahue, G. G. Malliaras, J. Rivnay and I. McCulloch, Adv. Funct. Mater., 2018, 28, 1706325 CrossRef.
- G. Tarabella, N. Coppedè, S. Iannotta, F. Cicoira, P. Kumar and C. Santato, Handbook of Organic Materials for Optical and (Opto)Electronic Devices: Properties and Applications, 2013, pp. 597–617 Search PubMed.
- A. M. Pappa, S. Inal, K. Roy, Y. Zhang, C. Pitsalidis, A. Hama, J. Pas, G. G. Malliaras and R. M. Owens, ACS Appl. Mater. Interfaces, 2017, 9, 10427–10434 CrossRef CAS PubMed.
- X. Strakosas, M. Bongo and R. M. Owens, J. Appl. Polym. Sci., 2015, 132, 1–14 CrossRef.
- J. Rivnay, R. M. Owens and G. G. Malliaras, Chem. Mater., 2014, 26, 679–685 CrossRef CAS.
- D. T. Simon, E. O. Gabrielsson, K. Tybrandt and M. Berggren, Chem. Rev., 2016, 116, 13009–13041 CrossRef CAS PubMed.
- J. C. Carlberg and O. Inganäs, J. Electrochem. Soc., 1997, 144, 5–8 CrossRef.
- A. Gölzhäuser, Phys. Chem. Chem. Phys., 2010, 12, 4273–4274 RSC.
- D. Moia, A. Giovannitti, A. A. Szumska, I. P. Maria, E. Rezasoltani, M. Sachs, M. Schnurr, P. R. F. Barnes, I. McCulloch and J. Nelson, Energy Environ. Sci., 2019, 12, 1349–1357 RSC.
- S. Muench, A. Wild, C. Friebe, B. Häupler, T. Janoschka and U. S. Schubert, Chem. Rev., 2016, 116, 9438–9484 CrossRef CAS PubMed.
- A. H. D. Macinnes, M. Druy, P. Nigrey, D. Nairns and A. McDiarmid, J. Chem. Soc., Chem. Commun., 1981, 317–319 RSC.
- T. B. Schon, B. T. McAllister, P. F. Li and D. S. Seferos, Chem. Soc. Rev., 2016, 45, 6345–6404 RSC.
- P. Gkoupidenis, D. A. Koutsouras and G. G. Malliaras, Nat. Commun., 2017, 8, 1–8 CrossRef PubMed.
- Y. Van De Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Nat. Electron., 2018, 1, 386–397 CrossRef.
- A. Giovannitti, D. T. Sbircea, S. Inal, C. B. Nielsen, E. Bandiello, D. A. Hanifi, M. Sessolo, G. G. Malliaras, I. McCulloch and J. Rivnay, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 12017–12022 CrossRef CAS PubMed.
- A. Giovannitti, I. P. Maria, D. Hanifi, M. J. Donahue, D. Bryant, K. J. Barth, B. E. Makdah, A. Savva, D. Moia, M. Zetek, P. R. F. Barnes, O. G. Reid, S. Inal, G. Rumbles, G. G. Malliaras, J. Nelson, J. Rivnay and I. McCulloch, Chem. Mater., 2018, 30, 2945–2953 CrossRef CAS PubMed.
- T.-H. Le, Y. Kim and H. Yoon, Polymers, 2017, 9, 150 CrossRef PubMed.
- L. Kergoat, B. Piro, M. Berggren, G. Horowitz and M. C. Pham, Anal. Bioanal. Chem., 2012, 402, 1813–1826 CrossRef CAS PubMed.
- Z. Yu, Y. Xia, D. Du and J. Ouyang, ACS Appl. Mater. Interfaces, 2016, 8, 11629–11638 CrossRef CAS PubMed.
- M. Yamashita, C. Otani, H. Okuzaki and M. Shimizu, Inst. Electr. Electron. Eng., 2011, 1, 3–6 Search PubMed.
- I. N. Hulea, H. B. Brom, A. J. Houtepen, D. Vanmaekelbergh, J. J. Kelly and E. A. Meulenkamp, Phys. Rev. Lett., 2004, 93, 16–19 CrossRef PubMed.
- J. D. Yuen, A. S. Dhoot, E. B. Namdas, N. E. Coates, M. Heeney, I. McCulloch, D. Moses and A. J. Heeger, J. Am. Chem. Soc., 2007, 129, 14367–14371 CrossRef CAS PubMed.
- C. Pitsalidis, A. M. Pappa, M. Porel, C. M. Artim, G. C. Faria, D. D. Duong, C. A. Alabi, S. Daniel, A. Salleo and R. M. Owens, Adv. Mater., 2018, 30, 1–8 CrossRef PubMed.
- A. M. Pappa, D. Ohayon, A. Giovannitti, I. P. Maria, A. Savva, I. Uguz, J. Rivnay, I. Mcculloch, R. M. Owens and S. Inal, Sci. Adv., 2018, 4, 1–8 Search PubMed.
- D. T. Duong, Y. Tuchman, P. Chakthranont, P. Cavassin, R. Colucci, T. F. Jaramillo, A. Salleo and G. C. Faria, Adv. Electron. Mater., 2018, 4, 1–7 Search PubMed.
- L. H. Jimison, S. A. Tria, D. Khodagholy, M. Gurfinkel, E. Lanzarini, A. Hama, G. G. Malliaras and R. M. Owens, Adv. Mater., 2012, 24, 5919–5923 CrossRef CAS PubMed.
- S. A. Tria, M. Ramuz, M. Huerta, P. Leleux, J. Rivnay, L. H. Jimison, A. Hama, G. G. Malliaras and R. M. Owens, Adv. Healthcare Mater., 2014, 3, 1053–1060 CrossRef CAS PubMed.
- H. Tang, F. Yan, P. Lin, J. Xu and H. L. W. Chan, Adv. Funct. Mater., 2011, 21, 2264–2272 CrossRef CAS.
- Q. Thiburce and A. J. Campbell, Adv. Electron. Mater., 2017, 3, 1600421 CrossRef.
- S. Zhang, P. Kumar, A. S. Nouas, L. Fontaine, H. Tang and F. Cicoira, APL Mater., 2015, 3, 1–8 Search PubMed.
- S. Holliday, J. E. Donaghey and I. McCulloch, Chem. Mater., 2014, 26, 647–663 CrossRef CAS.
- Z. Chen, M. J. Lee, R. Shahid Ashraf, Y. Gu, S. Albert-Seifried, M. Meedom Nielsen, B. Schroeder, T. D. Anthopoulos, M. Heeney, I. McCulloch and H. Sirringhaus, Adv. Mater., 2012, 24, 647–652 CrossRef CAS PubMed.
- J. Nightingale, J. Wade, D. Moia, J. Nelson and J. Kim, J. Phys. Chem. C, 2018, 122, 29129–29140 CrossRef CAS.
- I. D. Baikie, A. C. Grain, J. Sutherland and J. Law, Energy Procedia, 2014, 60, 48–56 CrossRef CAS.
- M. Skompska, Electrochim. Acta, 1998, 44, 357–362 CrossRef CAS.
- M. Skompska and A. Szkurlat, Electrochim. Acta, 2001, 46, 4007–4015 CrossRef CAS.
- A. M. Gaikwad, Y. Khan, A. E. Ostfeld and S. Pandya, Org. Electron., 2016, 30, 18–29 CrossRef CAS.
- G. Zhang, M. Mcbride, N. Persson, S. Lee, T. J. Dunn, M. F. Toney, Z. Yuan, Y. Kwon, P. Chu, B. Risteen and E. Reichmanis, Chem. Mater., 2017, 29, 7645–7652 CrossRef CAS.
- W. C. Tsoi, D. T. James, J. S. Kim, P. G. Nicholson, C. E. Murphy, D. D. C. Bradley, J. Nelson and J. S. Kim, J. Am. Chem. Soc., 2011, 133, 9834–9843 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Transfer curves with square root of IDS to estimate the VON values; UV-vis absorbance, air photoelectron spectroscopy and kelvin probe measurements of pristine and chemically doped P3HT; ERRS measurements demonstrating the volumetric effect. See DOI: 10.1039/d0tc02149k |
| This journal is © The Royal Society of Chemistry 2020 |