Polymer encapsulation and stabilization of molecular gel-based chiroptical information for strong, tunable circularly polarized luminescence film†
Abstract
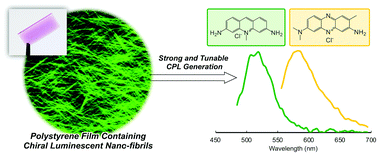
We demonstrate a strategic approach for a completely organic, chiroptical polymer film, exhibiting strong (glum = 0.01–0.05) and broad-band circularly polarized luminescence that can be realized by the stabilization of self-assembly-driven secondary chirality through polymer encapsulation.


 Please wait while we load your content...
Please wait while we load your content...