Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Huge enhancement of Sm2+ emission via Eu2+ energy transfer in a SrB4O7 pressure sensor†
Teng
Zheng
 a,
Marcin
Runowski
a,
Marcin
Runowski
 *a,
Przemysław
Woźny
*a,
Przemysław
Woźny
 a,
Stefan
Lis
a,
Stefan
Lis
 a and
Víctor
Lavín
a and
Víctor
Lavín
 b
b
aAdam Mickiewicz University, Faculty of Chemistry, Uniwersytetu Poznańskiego 8, 61-614 Poznań, Poland. E-mail: runowski@amu.edu.pl; Tel: +48618291778
bDepartamento de Física, MALTA Consolider Team and IUdEA, Universidad de La Laguna, Apdo. Correos 456, E-38200 San Cristóbal de La Laguna, Santa Cruz de Tenerife, Spain
First published on 2nd March 2020
Abstract
Taking advantage of the excellent pressure-sensing properties of the Sm2+ ion in the SrB4O7 crystal, we demonstrate an enormous enhancement of about 60 times in the emission intensity of Sm2+ ions when Eu2+ ions are also incorporated into the crystalline structure. This enhancement is induced by the energy transfer from Eu2+ to Sm2+ ions. The spectral position of the ultra-narrow and most intense 5D0 → 7F0 emission line in the material was correlated with pressure and successfully calibrated up to about 58 GPa. The material exhibits favorable pressure-sensing features, i.e. dλ/dp ≈ 0.29 nm GPa−1, negligible temperature-dependent shift, narrow and well-separated emission lines, and a strong luminescence signal. The samples also exhibit multicolor tunable luminescence from orange-red to amaranth, and to warm-white light, depending on the excitation wavelength used and dopant content in the matrix, allowing their potential application in white light emitting diode (LED) devices.
Introduction
Pressure shapes stars and planets, continents and oceans, and affects all aspects of our lives.1 Pressure also has a crucial influence on the physicochemical properties of materials. Compression of materials under high-pressure conditions has been widely utilized for the study of pressure-induced changes of physicochemical characteristics of substances, formations of new phases or materials under extreme conditions, spectroscopic and structural variation of the chemical compounds, etc.2–10 When trying to mimic these processes for scientific and industrial purposes in the laboratory using a high pressure anvil cell, a quick and precise determination of pressure is of paramount importance and, thanks to the transparency of diamonds and other gems in the visible range, optical pressure calibrants are often used.11In the case of a static high pressure experiment, the combination of a sample with a variety of hydrostatic pressure transmitting media (PTM) is a typical assemblage for diamond anvil cell (DAC) system, using the calibration of the line shift of the fluorescence at around 694 nm of Cr3+ ion in ruby, i.e. dλ/dp ≈ 0.35 nm GPa−1, for the pressure determination.12–15 However, due to the pressure-induced solidification of most of the commonly used PTMs and the subsequent loss of hydrostaticity in the sample's chamber,15–18 a large error occurs in the ruby spectral data analysis that significantly reduces the accuracy of the pressure determination under very high pressure conditions. This is because of the relatively broad (≈0.8 nm at ambient condition) emission bands of ruby and the strong overlapping of the ruby peaks (R1 and R2) under non-hydrostatic, high-pressure conditions. Moreover, the fluorescence line shift of ruby is strongly temperature-dependent, i.e. dλ/dT ≈ 0.007 nm K−1, which limits its pressure-sensing capability to the low-temperature range.
The SrB4O7:Sm2+ compound has been reported in many works as a splendid alternative to ruby as pressure gauge,18–21 thanks to its favorable features such as: (I) an isolated, narrow (≈0.2 nm) and intense 5D0 → 7F0 emission band (0–0 line) located at around 685 nm; (II) a large pressure-induced shift of the 0–0 line (≈0.25 nm GPa−1), comparable to that of Cr3+ in ruby, (III) a negligible temperature coefficient (10−4 nm K−1), and (IV) excellent thermal, chemical and structural stabilities. However, despite these benefits, and as it also happens to ruby, the inevitable decrease of Sm2+ luminescence intensity under pressure may limit the measurable pressure range and also may diminish the sensing accuracy.
The divalent lanthanide (Ln) ions are interesting luminescent centers because of the smaller energy gap between the 4fn ground and the first excited 4fn−15d configurations compared to the Ln3+ ions.22–29 This smaller energy difference increases the mixing of wavefunctions of the different configurations, i.e. increasing the 5d state opposite parity character of the 4f wavefunctions, that shorten the lifetime and increases the intensity of the luminescence of Ln2+ ions.30 The divalent europium ion (Eu2+) has many advantages, such as intense broad excitation and emission bands corresponding to the allowed 4f65d ↔ 4f7 transitions, relatively low reduction potential, relatively high stability, etc.31–35 Thanks to the large absorption in the UV region of Eu2+ in many host materials, this ion may exhibit excellent sensitization effects to other lanthanide ions.36–40 According to energy transfer (ET) mechanism,41–43 such a process occurring from sensitizer (Eu2+) to activator ions can effectively enhance emission of the luminophore. A host material based on the SrB4O7 crystal structure is a good choice for effective combination of sensitizing properties of Eu2+ ions with the targeted Sm2+ activator ions, to enhance luminescence performance of the system. This is because SrB4O7 host reveals excellent stabilization of both Eu2+ and Sm2+, even at high temperature in an oxidizing atmosphere. Moreover, the similar ionic radii of the dopant and host ions (REu2+ = 1.39 Å, RSm2+ = 1.41 Å and RSr2+ = 1.40 Å) provide less lattice distortion of the synthesized crystals.44–47
Many works have focused on the synthesis of different compounds doped with Sm2+ and other trivalent lanthanide ions, i.e. Ce3+, Er3+, Tm3+, etc., and their use for pressure sensing applications.25,26,48–53 However, there are no reports concerning signal enhancement of pressure sensors via the ET processes from Eu2+ to other Ln2+/3+ ions, which may significantly increase the emission intensity of various co-doped optical sensors, working under high pressure conditions. Herein, we report a huge enhancement of about 60 times in the emission intensity of Sm2+ caused by the Eu2+ → Sm2+ ET in the co-doped SrB4O7 compound synthesized by a simple solid-state method in air, and its application as a well-calibrated and accurate optical pressure sensor, which can work simultaneously under extreme conditions of pressure and temperature. In addition, we also demonstrate multi-color tunable emission from orange-red to warm white, depending on the concentration of Sm2+ and Eu2+ ions in the host and the excitation wavelength used.
Results and discussions
Structural and morphological properties
Information concerning the raw material used, synthesis and characterization are available in the ESI.† The powder X-ray diffraction (XRD) patterns (Fig. S1 in ESI†) of the SrB4O7:0.01Sm2+,xEu2+ (x = 0, 0.005, 0.01, 0.03, 0.05, 0.07 and 0.09) micro-particles fit well with the reference patterns (card no. 071-2191) from the ICDD standards database: orthorhombic SrB4O7 phase with space group Pmn21. The graphical representation of crystal structure is shown in Fig. 1a, where it is indicated that only one Sr2+ site exists in the structure of SrB4O7 host occupying a nonahedron (coordination number: 9) with local point symmetry C2v, and the cell parameters are: a = 4.431(4) Å, b = 10.707(10) Å, c = 4.237(4) Å, V = 200.810 Å3.The morphology of the synthesized products was inspected by a scanning electron microscopy (SEM), and the representative SEM images of SrB4O7:0.01Sm2+,xEu2+ samples (x = 0, 0.01, 0.03, 0.05) are shown in Fig. S2 (ESI†). The synthesized products are composed of irregular, nearly-spherical micro-particles (typical of inorganic borates), whose sizes range from approximately ∼0.2 to 2.0 μm. With increasing content of Eu2+, from 0 to 5 mol% (x = 0–0.05), the particle sizes vary slightly, which can be attributed to the similar ionic radii between the host and dopant ions.54–56 In Fig. S2e–k (ESI†), the representative elemental mapping results and the EDX spectrum of the SrB4O7:0.01Sm2+,0.03Eu2+ compound revealed that the obtained phosphor consist of Sr, B, O, Eu and Sm, and these elements are uniformly distributed in the prepared product over the whole particle volume.
Optical properties at ambient conditions
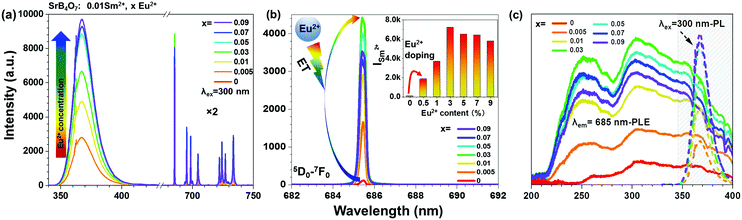
The photoluminescence (PL) emission and excitation spectra, as well as luminescence decay curves, of the samples obtained were recorded under the same experimental conditions, and were corrected for the apparatus response. In Fig. 1b, the excitation spectra are given for the SrB4O7:0.01Sm2+,0.03Eu2+ compound recorded monitoring the emission at 685 nm for Sm2+ (top) and 367 nm for Eu2+ (bottom). It was found that the excitation spectra of both Sm2+ and Eu2+ consist of two overlapped broad peaks with maxima at around ≈250 nm and ≈300 nm, which are due to the splitting of 5d orbital into the t2g and eg components.57 Upon UV light excitation at 300 nm (the highest peak in excitation spectra of Sm2+ in Fig. 1b), the PL emission spectrum of the SrB4O7:0.01Sm2+,0.03Eu2+ compound was examined. As shown in Fig. 1c, the emission spectrum consists of UV-violet and red bands. In the UV-violet region, the detected emission band can be deconvoluted into two peaks, i.e. the characteristic 4f65d → 4f7 transition of Eu2+ centered at 367 nm (broad band) and the rarely observed 4f7–4f7 line-type (narrow) transitions of Eu2+ centered at ≈362.1 and ≈362.7 nm.58–61 On the other hand, the red emissions of Sm2+, located in the range of ≈680–750 nm, correspond to 5D0 → 7F0 (685.4 nm), 5D0 → 7F1 (695.4, 698.6, 704.6 nm), 5D0 → 7F2 (722.1, 724.2, 727.0 and 733.5 nm) and they are associated with 4f6–4f6 intra-configurational transitions of Sm2+ ions. It is clearly seen that the 5D0 → 7F0 transition of Sm2+ exhibits its characteristic isolated, narrow and intense singlet band located at 685.4 nm. In the case of inevitable broadening of emission peaks under high pressure conditions, such a characteristic intensive, singlet and narrow 0–0 line of Sm2+ in a co-doped SrB4O7, reveals its superiority for high-pressure measurements, in comparison with the emissions bands of other transition metal ions (e.g. Mn4+, Ce3+, Er3+).53,62–64 This is attributed to the fact that the lowest emitting level and the ground state of the Sm2+ ion are singlets, i.e. non-degenerated multiplets with a total angular quantum momentum (J number) equal to zero. In addition, the 5D0 → 7F1 band presents three sharp peaks, while the 5D0 → 7F2 band presents only four out of five peaks. Hence, all the observed Sm2+ bands are split in a maximum of 2J + 1 Stark components, which further confirms only one Sm2+ local symmetry site (local point symmetry C2v) existing in the SrB4O7 structure.To better comprehend the feasible ET process and the luminescence mechanisms governing the generation of UV-violet and red emissions, the simplified energy level diagram of Eu2+ and Sm2+ ions is illustrated in Fig. 1d. With the excitation of UV light (from 210 to 360 nm), the Eu2+ ions can be excited from their ground 8S7/2 level to the excited 4f65d1, as well as 6P5/2 and 6P7/2 levels, and then radiatively relax to the ground state, accompanied with a broad UV-violet emission (5d → 4f) overlapping with line-type emission (4f7 → 4f7) of Eu2+. Meanwhile, the Sm2+ ions populating the 7F0 ground state can be excited to the 4f55d1 first excited configuration of Sm2+ when pumping at λex = 250–550 nm. Afterward, non-radiative (NR) decays occur, populating the lowest excited level 5D0, finally leading to a series of radiative 5D0 → 7FJ transitions, giving rise to the characteristic narrow bands of the red emission of Sm2+ ions. We assume that there is an energy transfer (ET) process between the Eu2+ and Sm2+ ions in the SrB4O7 host, which will be discussed in detail in the following paragraphs.
In order to explore the influence of Eu2+ ion concentration on the emission properties of the synthesized samples, the emission spectra exciting at λex = 300 nm of the samples as a function of the Eu2+ ion content are shown in Fig. 2a. It is clearly seen that all the emission spectra consist of UV-violet emission bands of Eu2+ ions and red emission lines from Sm2+ ions, except of the sample without Eu2+ ions (SrB4O7:0.01Sm2+) that only shows Sm2+ emission lines. With increasing the Eu2+ ion content from 0.5 to 9 mol% (x = 0.05–0.9), the shape of emission peaks scarcely varies, whereas the emission intensity and the Eu2+/Sm2+ band intensity ratios are greatly dependent on the Eu2+ doping content. In the UV-violet region, the emission intensity of Eu2+ maintains an upward tendency with increasing the Eu2+ doping content from 0 to 9 mol%. For detailed investigation of the sensitization effect of Eu2+ in the synthesized samples, the magnified emission spectra of the Sm2+: 0–0 line (in a narrow range) as a function of the Eu2+ doping content are shown in Fig. 2b. As disclosed, the Sm2+ emission intensity of the samples is highly dependent on the Eu2+ concentration and the best enhancement occurs for x = 0.03 (inset in Fig. 2b). Based on the integrated emission intensity of Sm2+ in the SrB4O7:0.01Sm2+,xEu2+ samples (see Table S1 in ESI†), it is clearly seen that the addition of even 0.5 mol% of Eu2+ results in the ≈16-times enhancement in emission intensity of Sm2+ compared to the sample without Eu2+ ions. As the Eu2+ content further increases, the enhancement demonstrates an upward tendency, exhibiting its optimal value, i.e. about 60 times enhancement, with 3 mol% of Eu2+. Afterwards, the emission intensity of Sm2+ ions starts to decrease when the doping concentration is over 3 mol%, probably due to the concentration quenching effects. Such a large increase in the intensity of Sm2+ emission in this material may be attributed to the ET phenomenon, occurring from Eu2+ to Sm2+ ions.
For the sake of studying the possible ET process from the Eu2+–Sm2+ ions, the emission spectra recorded at λex = 300 nm (Eu2+) and the excitation spectra at λem = 685 (Sm2+) of SrB4O7:0.01Sm2+,xEu2+ (x = 0–0.09) are shown in Fig. 2c, emphasizing their overlapping parts. It can be clearly seen that all the obtained samples have a very broad excitation band from 200 to over 400 nm, with the highest peak at around 300 nm, associated with Sm2+ ions that overlaps the Eu2+ emission band, located in the range from 345 to 400 nm. In fact, Eu2+ begins to emit even at lower wavelengths, however, due to the technical reasons, i.e. the necessity of using a long-pass (350 nm) filter, this emission is partially cutoff. The overlapping area indicates the resonant channel for ET from Eu2+ to Sm2+ ions, which occurs in the Sm2+–Eu2+ co-doped SrB4O7 system, leading to the enhancement of Sm2+ emission.65
The Commission Internationale de I’Eclairage (CIE) chromaticity diagram for the SrB4O7:0.01Sm2+,xEu2+ (x = 0–0.09) samples and the corresponding photographs of the samples under irradiation with a 254 nm UV lamp are shown in Fig. 3a. All the samples obtained are white powders in daylight. The presented colors in the CIE diagram (based on the emission spectra characteristics) agree with the luminescence photographs. As disclosed, the samples obtained exhibit multi-color tunable emission from orange-red to warm white, depending on the Eu2+ content in the phosphor. The corresponding emission spectra excited at 254 nm are shown in Fig. 3b. Moreover, as shown in Fig. 3c and d, the emission color of the best-enhanced sample (SrB4O7:0.01Sm2+,0.03Eu2+) can also be tuned depending on the excitation wavelength used. Noteworthy, the determined color coordinates of the Sm2+–Eu2+ co-doped SrB4O7 are very close to the white illumination (0.310, 0.316), e.g. 0.322, 0.306 (9 mol% of Eu2+) and 0.330, 0.325 (3 mol% of Eu2+), indicating that the phosphors obtained have potential application in white LED. All determined color coordinates as a function of the Eu2+ doping concentration and the excitation wavelength used are shown in Tables S2 and S3 in ESI,† respectively.
Optical properties at high pressure
To investigate the pressure-sensing abilities of Sm2+ in the Eu2+ co-doped SrB4O7 material, the best-enhanced sample, i.e. SrB4O7:0.01Sm2+,0.03Eu2+, was used in high-pressure measurements from ambient pressure up to about 58 GPa. The experimental setup used for the measurements of high-pressure luminescence is schematically presented in Fig. 4a. Due to technical reasons (availability of a high-power, focusable light source), the sample was excited at 280 nm, which is close to the optimal excitation wavelength. Technical details concerning measurements under high-pressure and sample preparation for DAC are presented in ESI.†The normalized emission spectra of the SrB4O7:0.01Sm2+,0.03Eu2+ material under pressure are shown in Fig. 4b. It can be clearly seen that, when the pressure increases, the red-shifts of the 5D0 → 7F0 and 5D0 → 7F1 emission peaks are observed. These shifts to lower energies under pressure are caused by an overall contraction of the 4fN ground configuration leading to a reduction in the energy difference between the ground state and the excited state of the 2S+1LJ multiplets.11,51 This phenomenon is related to decreasing coulomb and spin–orbit interactions under pressure, and it is called nephelauxetic effect,66 which can be accounted by a reduction of the free-ion parameters due to the increasing covalent character of the lanthanide–oxygen bonds, when interatomic distances decrease along with decreasing volume. Superimposed on the reduction in multiplets splitting, there is an increase in the splitting of the multiplets, ascribed to an increase of the crystal field interaction between the 4f electron of the lanthanide ion and the valence electrons of the oxygen anions of the first coordination shell, when interatomic distances decrease with pressure.10 This can be clearly observed in the splitting of the 5D0 → 7F1 emission peaks that increases from ≈9.2 nm (≈188 cm−1) at ambient pressure to ≈11.8 nm (≈230 cm−1) at 58.07 GPa. The emission spectra for all recorded pressure values are shown in Fig. S3(a–c) (ESI†).
The calibration curve for the spectral position of the Sm2+ 0–0 line as a function of pressure, in the compression and decompression cycles is presented in Fig. 4c. The peak centroid reversibly shifts from about 685.4 nm at ambient pressure to 702.3 nm at 58.07 GPa, which can be well fitted (R2 = 0.998) to a linear function. The calculated shift rate for this band is dλ/dT ≈ 0.29 nm GPa−1, which is close to the shifts of the SrB4O7:Sm2+ reported previously18–21,45 and also to that reported for ruby.12,15 All the exact dλ/dP values, and the initial and final spectral positions of the peak centroids (5D0 → 7F0 and 5D0 → 7F1 transitions) are presented in Table S4 in ESI.† The 0–0 line of Sm2+ in the SrB4O7:Eu2+–Sm2+ material is almost temperature-independent showing a relatively low thermal-quenching effect of dλ/dT ≈ −2.8 × 10−4 nm K−1, as reported for SrB4O7:Sm2+ in several papers,18–21,45 which we additionally confirmed for our compound (see Fig. S4 in ESI†). The low thermal-quenching effect presented, i.e. ≈75% of the initial emission intensity at 420 K (working temperature of LED devices), is beneficial not only for pressure sensing, but also for potential use in lighting applications.67,68
The broadening of the 5D0 → 7F0 and 5D0 → 7F1 band profiles together with the increase in pressure (volume reduction) is clearly seen in the presented emission spectra (Fig. 4b). At ambient conditions, the 0–0 line is extremely narrow, i.e. the full width at half maximum Γ ≈ 0.23 nm. It keeps an upward tendency with increasing pressure (see Fig. S3d, ESI†) and increases to ≈1.81 nm at 26.42 GPa, which is mainly due to the increasing non-hydrostaticity of the pressure transmitting medium used. Then, this peak undertakes a slower broadening, up to ≈2.27 nm at 58.07 GPa, with an average dΓ/dP ≈ 14.5 × 10−3 nm GPa−1.
Confirmation of ET from Eu2+ to Sm2+ in co-doped SrB4O7
In order to fully confirm the ET from Eu2+ to Sm2+, a series of SrB4O7 doped with a constant amount of Eu2+ and varied content of Sm2+ were synthesized. As shown in Fig. S5a (ESI†), the XRD patterns of the SrB4O7:ySm2+,0.05Eu2+ (y = 0.005, 0.01, 0.02, 0.03 and 0.05) micro-particles indicated that all phosphors were successfully synthesized. In Fig. S5b (ESI†), the emission spectra are shown for the excitation wavelength λex = 254 nm, indicating a gradual increase in the Sm2+ emission intensity and a deterioration of Eu2+ emission, as the Sm2+ doping content increases. In the inset in Fig. S5(b) (ESI†), the integrated emission intensity ratio of Sm2+/Eu2+ as a function of Sm2+ concentration shows an upward tendency. The CIE diagram for the synthesized phosphors at λex = 254 nm, i.e. the comparison of the emission color between different doping content of Sm2+ is shown in Fig. S5c (ESI†). The colors presented in this CIE diagram agree with the luminescence photographs. The corresponding color coordinates are listed in Table S5 (ESI†). Accordingly, the corresponding emission color can be tuned from orange-red to amaranth depending on the Sm2+ content in the SrB4O7:ySm2+,0.05Eu2+ samples.The photoluminescence decay curves of the as-prepared SrB4O7:ySm2+,0.05Eu2+ (y = 0.005–0.05) samples are presented in Fig. S5d (ESI†). The luminescence decay curves of these phosphors were recorded at λem = 367 nm for the Eu2+ ion after the excitation at 300 nm. The decay curves of Eu2+ emission show a non-exponential character (due to the high doping concentration). However, to analyze the tendency of luminescence lifetimes as a function of Sm2+ content, and confirm the Eu2+ → Sm2+ ET phenomenon, the decay profiles were fitted (R2 > 0.999) to the bi-exponential function:
I = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−x/τ1) + A2 exp(−x/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−x/τ2) exp(−x/τ2) | (1) |
| Sample (% Sm2+) | Eu2+ (4f65d1 → 4f7) | |||||
|---|---|---|---|---|---|---|
| τ 1 (μs) | A 1 (%) | τ2 (μs) | A 2 (%) | τ (μs) | R 2 | |
| 0.5 | 0.668 ± 0.006 | 0.117 | 2.616 ± 0.002 | 0.883 | 2.388 | >0.999 |
| 1 | 0.570 ± 0.006 | 0.142 | 2.500 ± 0.004 | 0.858 | 2.226 | >0.999 |
| 2 | 0.559 ± 0.002 | 0.189 | 2.326 ± 0.002 | 0.811 | 1.992 | >0.999 |
| 3 | 0.461 ± 0.002 | 0.249 | 2.227 ± 0.002 | 0.751 | 1.787 | >0.999 |
| 5 | 0.398 ± 0.002 | 0.272 | 2.117 ± 0.003 | 0.728 | 1.649 | >0.999 |
Conclusions
Incorporating Eu2+ ions into the crystal structure of the commonly used optical pressure sensor SrB4O7:Sm2+ (temperature-independent gauge), we have demonstrated a huge enhancement of about 60 times on the Sm2+ emission, induced by energy transfer processes from Eu2+ to Sm2+ in the co-doped material. The samples obtained exhibit color-tunable luminescence from orange-red to amaranth, and to warm white depending on the Sm2+ and Eu2+ concentrations in the matrix and the excitation wavelength. The most intense and extremely narrow (≈0.2 nm) 0–0 line of the Sm2+ (around 685 nm) in the best-enhanced sample (SrB4O7: 1 mol% Sm2+, 3 mol% Eu2+) was correlated with pressure and successfully calibrated up to about 58 GPa. The emission line used for pressure sensing exhibit a large and linear red-shift under pressure, i.e. dλ/dp ≈ 0.29 nm GPa−1, a negligible temperature-induced band-shift and relatively low thermal quenching of luminescence. The energy transfer processes from Eu2+ to Sm2+ ions were confirmed by luminescence spectroscopy. Such favorable features can significantly extend the measurable pressure range, along with very high accuracy of pressure determination, even under high temperature conditions, and the material can be applied in white light emitting diode (LED) devices.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Polish National Science Centre, grant no. 2016/23/D/ST4/00296 and 2016/21/B/ST5/00110, The Ministerio de Economía y Competitividad (MINECO) under the Spanish National Program of Materials (MAT2016-75586-C4-4-P), The Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI) (ProID2017010078), and grant no. POWR.03.02.00-00-I023/17 and POWR.03.02.00-00-i020/17 co-financed by the European Union through the European Social Fund under the Operational Program Knowledge Education Development, and by The EU-FEDER funds. M. R. is a recipient of the Bekker Programme scholarship supported by the Polish National Agency for Academic Exchange.References
- An Introduction to High-Pressure Science and Technology, ed. J. M. Recio, J. M. Menendez and A. Otero de la Roza, CRC Press, Boca Raton, 2016 Search PubMed.
- M. Runowski, S. Sobczak, J. Marciniak, I. Bukalska, S. Lis and A. Katrusiak, Nanoscale, 2019, 11, 8718–8726 RSC.
- F. Bai, K. Bian, X. Huang, Z. Wang and H. Fan, Chem. Rev., 2019, 119, 7673–7717 CrossRef CAS PubMed.
- M. Somayazulu, P. Dera, A. F. Goncharov, S. A. Gramsch, P. Liermann, W. Yang, Z. Liu, H. K. Mao and R. J. Hemley, Nat. Chem., 2010, 2, 50–53 CrossRef CAS PubMed.
- A. Cantaluppi, M. Buzzi, G. Jotzu, D. Nicoletti, M. Mitrano, D. Pontiroli, M. Riccò, A. Perucchi, P. Di Pietro and A. Cavalleri, Nat. Phys., 2018, 14, 837–841 Search PubMed.
- Z. Ma, Z. Liu, S. Lu, L. Wang, X. Feng, D. Yang, K. Wang, G. Xiao, L. Zhang, S. A. T. Redfern and B. Zou, Nat. Commun., 2018, 9, 4506 Search PubMed.
- W. Kim, M. S. Jung, S. Lee, Y. J. Choi, J. K. Kim, S. U. Chai, W. Kim, D. G. Choi, H. Ahn, J. H. Cho, D. Choi, H. Shin, D. Kim and J. H. Park, Adv. Energy Mater., 2018, 8, 1702369 Search PubMed.
- J. Song, G. Fabbris, W. Bi, D. Haskel and J. S. Schilling, Phys. Rev. Lett., 2018, 121, 037004 CrossRef CAS PubMed.
- L. Zhang, C. Liu, L. Wang, C. Liu, K. Wang and B. Zou, Angew. Chem., Int. Ed., 2018, 57, 11213–11217 CrossRef CAS PubMed.
- T. Yin, Y. Fang, W. K. Chong, K. T. Ming, S. Jiang, X. Li, J. L. Kuo, J. Fang, T. C. Sum, T. J. White, J. Yan and Z. X. Shen, Adv. Mater., 2018, 30, 1705017 CrossRef PubMed.
- Th. Tröster, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschnidner, Jr., J.-C. G. Bünzli and V. K. Pecharsky, Elsevier, North-Holland, 2003, vol. 33, pp. 515–589 Search PubMed.
- P. M. Bell, H. K. Mao and K. Goettel, Science, 1984, 226, 542–544 CrossRef CAS PubMed.
- J. A. Xu, H. K. Mao and P. M. Bell, Science, 1986, 232, 1404–1406 CrossRef CAS PubMed.
- K. Syassen, High Press. Res., 2008, 28, 75–126 CrossRef CAS.
- G. J. Piermarini, S. Block, J. D. Barnett and R. A. Forman, J. Appl. Phys., 1975, 46, 2774–2780 CrossRef CAS.
- R. J. Angel, M. Bujak, J. Zhao, G. D. Gatta and S. D. Jacobsen, J. Appl. Crystallogr., 2007, 40, 26–32 CrossRef CAS.
- R. J. Angel, Transformation Processes in Minerals, De Gruyter, Mouton, 2019, vol. 39, pp. 85–104 Search PubMed.
- F. Datchi, R. LeToullec and P. Loubeyre, J. Appl. Phys., 1997, 81, 3333–3339 CrossRef CAS.
- C. Zhao, H. Li, Y. Wang, J. Jiang and Y. He, High Press. Res., 2017, 37, 18–27 CrossRef CAS.
- S. V. Rashchenko, A. Kurnosov, L. Dubrovinsky and K. D. Litasov, J. Appl. Phys., 2015, 117, 2–7 CrossRef.
- Q. Jing, Q. Wu, L. Liu, J. Xu, Y. Bi, Y. Liu, H. Chen, S. Liu, Y. Zhang, L. Xiong, Y. Li and J. Liu, J. Appl. Phys., 2013, 113, 023507 CrossRef.
- T. Zheng, L. Luo, P. Du, A. Deng and W. Li, J. Eur. Ceram. Soc., 2018, 38, 575–583 CrossRef CAS.
- Q. Dong, J. Cui, Y. Tian, F. Yang, H. Ming, F. Du, J. Peng and X. Ye, J. Lumin., 2019, 212, 146–153 CrossRef CAS.
- T. Zheng and L. Luo, Ceram. Int., 2018, 44, 12670–12675 CrossRef CAS.
- M. Runowski, Handbook of Nanomaterials in Analytical Chemistry, Elsevier, 2020, pp. 227–273 Search PubMed.
- M. Runowski, P. Woźny, S. Lis, V. Lavín and I. R. Martín, Adv. Mater. Technol., 2020, 1901091 CrossRef.
- H. Li, Q. Li and Z. Xu, J. Mater. Chem. C, 2019, 7, 2880–2885 RSC.
- H. Ji, L. Wang, M. S. Molokeev, N. Hirosaki, R. Xie, Z. Huang, Z. Xia, O. M. Ten Kate, L. Liu and V. V. Atuchin, J. Mater. Chem. C, 2016, 4, 6855–6863 RSC.
- V. V. Atuchin, A. S. Aleksandrovsky, O. D. Chimitova, T. A. Gavrilova, A. S. Krylov, M. S. Molokeev, A. S. Oreshonkov, B. G. Bazarov and J. G. Bazarova, J. Phys. Chem. C, 2014, 118, 15404–15411 CrossRef CAS.
- Y. Shen and K. L. Bray, Mater. Sci. Forum, 1999, 315–317, 243–248 CAS.
- H. Fang, G. Qiu, J. Li and X. Wang, J. Alloys Compd., 2018, 763, 267–272 CrossRef CAS.
- W. J. Evans, Coord. Chem. Rev., 2000, 206–207, 263–283 CrossRef CAS.
- R. Shi, L. Ning, Y. Huang, Y. Tao, L. Zheng, Z. Li and H. Liang, ACS Appl. Mater. Interfaces, 2019, 11, 9691–9695 CrossRef CAS PubMed.
- Z. Xia, Y. Zhang, M. S. Molokeev and V. V. Atuchin, J. Phys. Chem. C, 2013, 117, 20847–20854 CrossRef CAS.
- Z. Wang, Z. Xia, M. S. Molokeev, V. V. Atuchin and Q. Liu, Dalton Trans., 2014, 43, 16800–16804 RSC.
- H. Lai, J. Zhang, D. Hou, H. Guan and X. Ye, Ceram. Int., 2018, 44, 15072–15078 CrossRef CAS.
- L. Dong, L. Zhang, W. Lü, B. Shao, S. Zhao and H. You, Dalton Trans., 2019, 48, 3028–3037 RSC.
- D. Liu, Y. Jin, Y. Lv, G. Ju, C. Wang, L. Chen, W. Luo and Y. Hu, J. Am. Ceram. Soc., 2018, 101, 5627–5639 CrossRef CAS.
- M. Shi, C. Zhu, M. Lu, X. Meng and M. Wei, J. Am. Ceram. Soc., 2018, 101, 5461–5468 CrossRef CAS.
- W. Wolszczak, K. W. Krämer and P. Dorenbos, J. Lumin., 2020, 222, 117101 CrossRef CAS.
- H. Dong, L. D. Sun and C. H. Yan, Chem. Soc. Rev., 2015, 44, 1608–1634 RSC.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef.
- J. Xue, X. Wang, J. H. Jeong and X. Yan, Phys. Chem. Chem. Phys., 2018, 20, 11516–11541 RSC.
- T. Zheng, M. Runowski, P. Woźny and S. Lis, J. Alloys Compd., 2020, 822, 1–9 Search PubMed.
- Z. Cao, X. Wei, L. Zhao, Y. Chen and M. Yin, ACS Appl. Mater. Interfaces, 2016, 8, 34546–34551 CrossRef CAS PubMed.
- J. Sun, J. Zhu, X. Liu and H. Du, J. Rare Earths, 2012, 30, 1084–1087 CrossRef CAS.
- P. Solarz, M. Karbowiak, M. Głowacki, M. Berkowski, R. Diduszko and W. Ryba-Romanowski, J. Alloys Compd., 2016, 661, 419–427 CrossRef CAS.
- S. Kobyakov, A. Kamińska, A. Suchocki, D. Galanciak and M. Malinowski, Appl. Phys. Lett., 2006, 88, 234102 CrossRef.
- Q. Jing, Q. Wu, L. Liu, J. Xu, Y. Bi, Y. Liu, H. Chen, S. Liu, Y. Zhang, L. Xiong, Y. Li and J. Liu, J. Appl. Phys., 2013, 113, 023507 CrossRef.
- M. Runowski, A. Shyichuk, A. Tymiński, T. Grzyb, V. Lavín and S. Lis, ACS Appl. Mater. Interfaces, 2018, 10, 17269–17279 CrossRef CAS PubMed.
- M. Runowski, P. Woźny, V. Lavín and S. Lis, Sens. Actuators, B, 2018, 273, 585–591 CrossRef CAS.
- P. Woźny, M. Runowski and S. Lis, J. Lumin., 2019, 209, 321–327 CrossRef.
- M. Runowski, J. Marciniak, T. Grzyb, D. Przybylska, A. Shyichuk, B. Barszcz, A. Katrusiak and S. Lis, Nanoscale, 2017, 9, 16030–16037 RSC.
- X. Feng, D. C. Sayle, Z. L. Wang, M. S. Paras, B. Santora, A. C. Sutorik, T. X. T. Sayle, Y. Yang, Y. Ding, X. Wang and Y. Her, Science, 2006, 312, 1504–1507 CrossRef CAS PubMed.
- X. Wang, Y. Wang, J. Yu, Y. Bu and X. Yan, Opt. Express, 2018, 26, 21950 CrossRef CAS PubMed.
- D. Chen, Y. Yu, F. Huang, P. Huang, A. Yang and Y. Wang, J. Am. Chem. Soc., 2010, 132, 9976–9978 CrossRef CAS PubMed.
- R. Stefani, A. D. Maia, E. E. S. Teotonio, M. A. F. Monteiro, M. C. F. C. Felinto and H. F. Brito, J. Solid State Chem., 2006, 179, 1086–1092 CrossRef CAS.
- W. Liu, L. Liu, Y. Wang, L. Chen, J. A. McLeod, L. Yang, J. Zhao, Z. Liu, J. Diwu, Z. Chai, T. E. Albrecht-Schmitt, G. Liu and S. Wang, Chem. – Eur. J., 2016, 22, 11170–11175 CrossRef CAS PubMed.
- R. A. Hewes and M. V. Hoffman, J. Lumin., 1971, 3, 261–280 CrossRef CAS.
- M. V. Hoffman, J. Electrochem. Soc., 1972, 119, 905–909 CrossRef CAS.
- S. G. Jantz, F. Pielnhofer, L. van Wüllen, R. Weihrich, M. J. Schäfer and H. A. Höppe, Chem. – Eur. J., 2018, 24, 443–450 CrossRef CAS PubMed.
- R. Verstraete, H. F. Sijbom, J. J. Joos, K. Korthout, D. Poelman, C. Detavernier and P. F. Smet, ACS Appl. Mater. Interfaces, 2018, 10, 18845–18856 CrossRef CAS PubMed.
- P. Cai, X. Wang and H. J. Seo, Phys. Chem. Chem. Phys., 2018, 20, 2028–2035 RSC.
- M. Runowski, P. Woźny, N. Stopikowska, Q. Guo and S. Lis, ACS Appl. Mater. Interfaces, 2019, 11, 4131–4138 CrossRef CAS PubMed.
- S. Stojadinović and R. Vasilić, Mater. Lett., 2019, 234, 9–12 CrossRef.
- R. Reisfeld and C. K. Jørgensen, Lasers and Excited States of Rare Earths, 2012 Search PubMed.
- Q. Guo, Q. Wang, L. Jiang, L. Liao, H. Liu and L. Mei, Phys. Chem. Chem. Phys., 2016, 18, 15545–15554 RSC.
- Q. Guo, C. Zhao, L. Liao, S. Lis, H. Liu, L. Mei and Z. Jiang, J. Am. Ceram. Soc., 2017, 100, 2221–2231 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; XRD data; SEM images; EDX mapping and spectra; emission spectra; FWHM in pressure; temperature shift; luminescence decay curves and luminescence lifetime; CIE diagram. See DOI: 10.1039/d0tc00463d |
| This journal is © The Royal Society of Chemistry 2020 |