Non-fullerene small molecule acceptors with three-dimensional thiophene/selenophene-annulated perylene diimides for efficient organic solar cells†
Received
18th January 2020
, Accepted 30th March 2020
First published on 31st March 2020
Abstract
New three-dimensional non-fullerene small molecule acceptors were synthesized with the structure of a spiro core, 4,4′-spirobi[cyclopenta[2,1-b;3,4-b′]dithiophene] (SCPDT) linked with S/Se fused perylene diimides (PDIs). The three-dimensional structure suppresses the aggregation of PDIs and the formation of excessively large crystalline domains. The well-conjugated structure and electron-rich property of the spiro core give rise to high electron mobility, making for more balanced carrier transport. The introduction of S/Se aromatic rings leads to appropriate energy levels and more twisted molecular configurations. Organic solar cells based on the PBDB-T-S:SCPDT-PDI4-S bulk heterojunction exhibit a high power conversion efficiency of 6.95% along with a high Voc of 1.00 V, a Jsc of 11.72 mA cm−2 and a fill factor of 59.27%.
Introduction
In past decades, bulk heterojunction (BHJ) organic solar cells (OSCs) have attracted extensive attention owing to their attractive characteristics, for instance, low cost, flexibility in their fabrication, large-area printing production and environmentally friendly nature.1–9 Polymer materials as donors and fullerene or non-fullerene materials as acceptors make up the active layers of the OSCs. Fullerene derivatives such as PC61BM ([6,6]-phenyl-C61-butyric acid methyl ester) and PC71BM ([6,6]-phenyl-C71-butyric acid methyl ester) have undergone remarkable development in the last few years and power conversion efficiencies (PCEs) of fullerene-based BHJ OSCs of over 11% have been reported.10–12 Despite fullerene derivatives playing a significant role in the development of OSCs, their weaknesses are obvious, for example the fabrication of fullerene is costly and energy level tuning is not easy.13,14 Hence, non-fullerene acceptors have been paid much attention in the development of OSCs. Compared with fullerene, non-fullerene acceptors have advantageous characteristics, such as tunable energy levels, appropriate optical absorption, good chemical stability and excellent morphology stability.15–18 To date, a series of high-performance non-fullerene acceptors have been intensively developed and studied, such as perylene diimide (PDI),19 ITIC,14 Y620 and their derivatives.16–28 Especially, thanks to their strong absorption in the near-infrared region, high electron mobility and suitable energy levels, OSCs based on Y6 or its derivative acceptors have already achieved PCEs of over 17%,29–31 which are to date the highest efficiencies for single-junction OSCs. Meanwhile, PDI-based acceptors have also been subject to much exploration, owing to their fascinating properties such as easy accessibility, intense absorption in the visible region, high electron affinity and mobility, and excellent photochemical stability.23,32–35 The PCEs of OSCs with PDI derivative acceptors have exceeded 10%.24 However, PDI has a large planar structure and strong intermolecular π–π stacking, leading to the formation of excessively large crystalline domains, which may result in considerable phase separation in the active layer and thus limit the device performance.35,36 So as to suppress the tendency to aggregate, many methods have been explored, such as the construction of twisted PDI dimers and three-dimensional structured oligomers. In addition, S,37–39 Se17,22 and N40 heteroatoms have been incorporated at the bay positions of PDI units to gain more twisted structures and higher lowest unoccupied molecular orbital (LUMO) levels. Although great progress has been made in acceptors based on multidimensional PDIs, most of the previous work has focused on the structure of twisted PDI dimers. In comparison, besides suppressing the aggregation, these three-dimensional structures can further promote isotropic charge transport, which is favorable to the device performance. Therefore, it is pertinent to make high performance PDI-based acceptors by adopting the three-dimensional structures.
In this study, we report two new propeller-shaped small molecule acceptors (SMAs) named SCPDT-PDI4-S and SCPDT-PDI4-Se, based on a spiro core 4,4′-spirobi[cyclopenta[2,1-b;3,4-b′]dithiophene] (SCPDT) linked with S/Se fused PDIs. The spiro core SCPDT possesses an orthogonal molecular conformation with two planar cyclopenta[2,1-b;3,4-b′]dithiophene (CPDT) units connected via a spiro sp3 carbon, and shows unique optical and electrochemical properties due to the use of thiophene as the central aromatic system to provide the π-electron conjugation.41,42 Owing to the well-conjugated structure and electron-rich property of two CPDTs,43 these two SMAs are expected to possess strong intramolecular charge transfer characteristics and thus broad low-energy optical transitions and high carrier mobilities. The introduction of S/Se aromatic rings has an important effect on the energy levels and molecular configurations, as well as carrier mobilities. By using SCPDT-PDI4-S as the acceptor and the polymer PBDB-T-S as the donor, the OSCs show a higher PCE of 6.95% compared with that of 4.26% for SCPDT-PDI4-Se-based counterparts, which is attributed to the combined properties of appropriate energy level, balanced carrier mobility and appropriate phase separation in the active layer.
Results and discussion
Synthesis and characterization
The synthetic routes of the two new acceptors are illustrated in Scheme 1 and Scheme S1 (ESI†). The synthesis of PDI-S uses methods borrowed from previous literature,37 by which PDI-S was obtained through the reaction of the compound PDI-NO2 with S in N-methyl-2-pyrrolidone (NMP) solvent. Then, it was reacted with liquid bromine at room temperature to produce monomer PDI-S-Br. Afterwards, SCPDT-PDI4-S was yielded via Stille coupling reaction between 4Sn-SCPDT and the compound PDI-S-Br. The synthetic route of SCPDT-PDI4-Se was similar to that of SCPDT-PDI4-S. The compounds were characterized by NMR spectroscopy and mass spectrometry (Fig. S5–S8, ESI†). Both SCPDT-PDI4-S and SCPDT-PDI4-Se show good thermal stabilities (Fig. S4, ESI†), with 5% weight loss at 368.75 °C and 370.85 °C, respectively. They show excellent solubility in common organic solvents such as chloroform and chlorobenzene, which is mainly due to their 3D molecular structure and branched alkyl side chains.
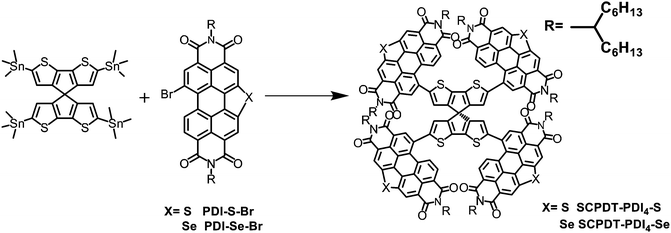 |
| | Scheme 1 Synthesis routes of SCPDT-PDI4-S and SCPDT-PDI4-Se. | |
Optical and electrochemical properties
The UV-visible absorption spectra of SCPDT-PDI4-S and SCPDT-PDI4-Se in solutions and thin films are illustrated in Fig. 1 and the corresponding data are summarized in Table 1. As shown in Fig. 1a, the two acceptors show similar absorption profiles in dichloromethane solution (10−5 M), while the spectrum of SCPDT-PDI4-S exhibits a slight blue shift. In the range of 400–600 nm, SCPDT-PDI4-S and SCPDT-PDI4-Se exhibit high absorption with the peaks at 498 and 503 nm, respectively. In Fig. 1b, it can be seen that the absorption spectra of the films are quite similar to those of the solutions, indicating a weak intermolecular aggregation of the two acceptors in the solid state. The optical bandgaps (Eoptg) of SCPDT-PDI4-S and SCPDT-PDI4-Se are estimated to be 1.87 and 1.84 eV, respectively, from the empirical formula Eoptg = 1240/λonset. It is easy to see in Fig. 1b that the combination of the absorbances of donor molecule PBDB-T-S and these two acceptors covers the visible spectrum well. And in comparison, owing to the blue shift, the spectrum of SCPDT-PDI4-S is expected to make a better combination with that of the PBDB-T-S donor. To further verify these, the absorption spectra of SCPDT-PDI4-S and SCPDT-PDI4-Se blended with PBDB-T-S (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) are characterized, which confirm a full harvest of light in the visible spectrum, as shown in Fig. S1 (ESI†). As expected, the PBDB-T-S:SCPDT-PDI4-S blend shows superior absorbance compared with PBDB-T-S:SCPDT-PDI4-Se one.
1, w/w) are characterized, which confirm a full harvest of light in the visible spectrum, as shown in Fig. S1 (ESI†). As expected, the PBDB-T-S:SCPDT-PDI4-S blend shows superior absorbance compared with PBDB-T-S:SCPDT-PDI4-Se one.
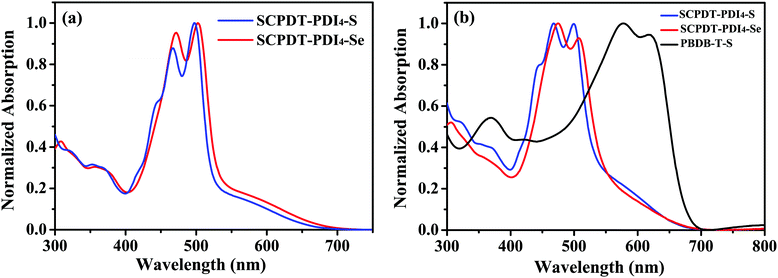 |
| | Fig. 1 (a) The normalized UV-vis absorption spectra of SCPDT-PDI4-S and SCPDT-PDI4-Se in dichloromethane solution (10−5 M). (b) The normalized absorption spectra of SCPDT-PDI4-S, SCPDT-PDI4-Se and PBDB-T-S thin films. | |
Table 1 Basic properties of SCPDT-PDI4-S and SCPDT-PDI4-Se
| Acceptor |
λ
max (nm) |
λ
onset (nm) |
E
optg (eV) |
HOMO (eV) |
LUMO (eV) |
| SCPDT-PDI4-S |
499 |
663 |
1.87 |
−5.62 |
−3.75 |
| SCPDT-PDI4-Se |
509 |
674 |
1.84 |
−5.58 |
−3.74 |
Cyclic voltammogram (CV) measurements were carried out in dichloromethane solution (Fig. S2, ESI†). The LUMO and highest occupied molecular orbital (HOMO) levels were estimated to be −3.75/−5.62 eV and −3.74/−5.58 eV for SCPDT-PDI4-S and SCPDT-PDI4-Se, respectively, according to the reduction onset potential and optical bandgap (Eoptg) (Table 1). The LUMO levels of SCPDT-PDI4-S and SCPDT-PDI4-Se are almost the same and show a rise in comparison with that of SCPDT-PDI4 (−3.80 eV),42 which can be attributed to the introduction of S/Se aromatic ring with electron-donating ability.17,39 The energy offset between LUMO of SCPDT-PDI-S (−3.75 eV) and HOMO of PBDB-T-S (−5.35 eV) is 1.6 eV; therefore a high Voc can be reasonably expected.
Theoretical analysis
In order to get the optimal 3D molecular geometries, density functional theory (DFT) calculations were performed at the B3LYP/6-31G(d) level.44 For convenience of calculation, we used methyl to replace long branched alkyl chains. Both SCPDT-PDI4-S and SCPDT-PDI4-Se show 3D nonplanar configurations, which should contribute to suppressing aggregation tendency and intermolecular stacking. As shown in Fig. 2, the LUMOs of SCPDT-PDI4-S and SCPDT-PDI4-Se are mainly located on the SCPDT core, while the HOMOs are distributed on peripheral PDIs, which implies a polarization in the excited state. The LUMO/HOMO levels of SCPDT-PDI4-S and SCPDT-PDI4-Se are −3.52/−5.77 eV and −3.49/−5.77 eV, respectively, from DFT calculations, which are consistent with the experimental values from the CV measurements. Moreover, the four dihedral angles between CPDT and the PDI unit are 81.88°, 77.10°, 78.07° and 80.07° for SCPDT-PDI4-S, and 81.20°, 74.26°, 74.42° and 80.97° for SCPDT-PDI4-Se (see Fig. 2). Compared with the value of 59° for all these dihedral angles in SCPDT-PDI4,34 this remarkable increase clearly indicates that the introduction of an S/Se aromatic ring (especially thiophene) leads to a more twisted molecular structure, which is beneficial to the suppression of molecular aggregation and the blending of donor and acceptor in the active layer.
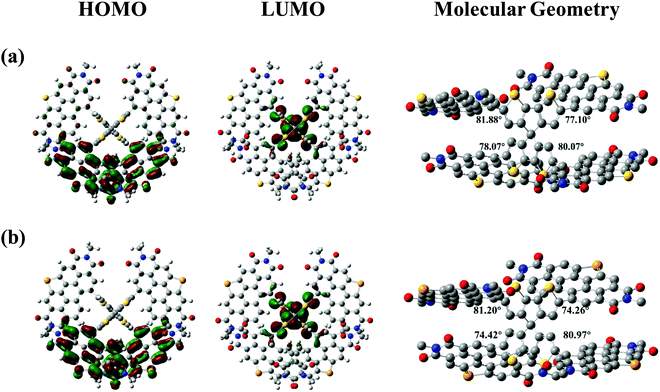 |
| | Fig. 2 HOMOs, LUMOs and optimized molecular geometries of (a) SCPDT-PDI4-S and (b) SCPDT-PDI4-Se calculated by DFT at the B3LYP/6-31G(d) level. | |
Photovoltaic properties
OSCs using SCPDT-PDI4-S and SCPDT-PDI4-Se as acceptors were fabricated with the conventional architecture of ITO/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/PBDB-T-S:acceptors/2,9-bis((3-(dimethylamino)propyl)anthra[2,1,9-def:6,5,10-def]diisoquinoline-1,3,8,10(2H,9H)-tetraone) (PDIN)/Al, as shown in Fig. 3 and Fig. S3 (ESI†). The wide bandgap polymer PBDB-T-S was selected as the donor owing to its complementary absorbance and matched energy levels with SCPDT-PDI4-S and SCPDT-PDI4-Se. In our experiment, the optimal donor–acceptor weight ratio was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a total concentration of 20 mg mL−1 in dichlorobenzene. The current density–voltage (J–V) characteristics of the devices are shown in Fig. 4a and the parameters are summarized in Table 2. The PBDB-T-S:SCPDT-PDI4-S-based device (SCPDT-PDI4-S device) achieves a high PCE of 6.95% along with a high Voc of 1.00 V, Jsc of 11.72 mA cm−2 and fill factor (FF) of 59.27%, while the PBDB-T-S:SCPDT-PDI4-Se-based device (SCPDT-PDI4-Se device) has a PCE of 4.26%, along with a Voc of 0.97 V, Jsc of 8.70 mA cm−2 and FF of 50.55%. It can be easily seen the SCPDT-PDI4-S device shows the much higher Jsc, which can be ascribed to the more complementary absorbance of SCPDT-PDI4-S with the donor molecule. In addition, the larger FF of the SCPDT-PDI4-S device is believed to arise from the more favorable morphology of PBDB-T-S:SCPDT-PDI4-S film compared with that of PBDB-T-S:SCPDT-PDI4-Se film, which would reduce the impedance at the interface. Note that the SCPDT-PDI4-S device also shows a higher efficiency than the SCPDT-PDI4-based device with the different polymer donor PTB7-Th (PCE = 6.31%, without additive), as reported in the literature.42 In our study, the donor PTB7-Th paired with SCPDT-PDI4-S and SCPDT-PDI4-Se acceptors was also tried but led to a relatively low performance.
1, with a total concentration of 20 mg mL−1 in dichlorobenzene. The current density–voltage (J–V) characteristics of the devices are shown in Fig. 4a and the parameters are summarized in Table 2. The PBDB-T-S:SCPDT-PDI4-S-based device (SCPDT-PDI4-S device) achieves a high PCE of 6.95% along with a high Voc of 1.00 V, Jsc of 11.72 mA cm−2 and fill factor (FF) of 59.27%, while the PBDB-T-S:SCPDT-PDI4-Se-based device (SCPDT-PDI4-Se device) has a PCE of 4.26%, along with a Voc of 0.97 V, Jsc of 8.70 mA cm−2 and FF of 50.55%. It can be easily seen the SCPDT-PDI4-S device shows the much higher Jsc, which can be ascribed to the more complementary absorbance of SCPDT-PDI4-S with the donor molecule. In addition, the larger FF of the SCPDT-PDI4-S device is believed to arise from the more favorable morphology of PBDB-T-S:SCPDT-PDI4-S film compared with that of PBDB-T-S:SCPDT-PDI4-Se film, which would reduce the impedance at the interface. Note that the SCPDT-PDI4-S device also shows a higher efficiency than the SCPDT-PDI4-based device with the different polymer donor PTB7-Th (PCE = 6.31%, without additive), as reported in the literature.42 In our study, the donor PTB7-Th paired with SCPDT-PDI4-S and SCPDT-PDI4-Se acceptors was also tried but led to a relatively low performance.
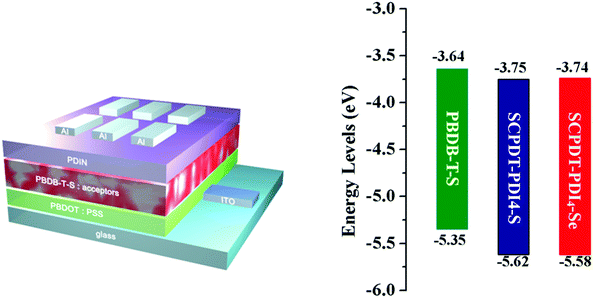 |
| | Fig. 3 Device configuration of the OSCs and energy levels of PBDB-T-S, SCPDT-PDI4-S and SCPDI-PDI4-Se. | |
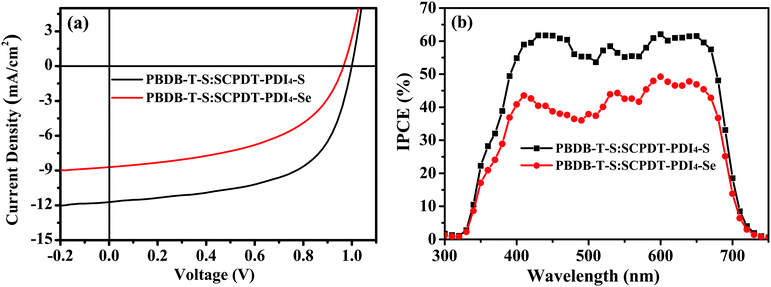 |
| | Fig. 4 (a) J–V curves and (b) IPCE spectra of the OSCs with PBDB-T-S:SCPDT-PDI4-S and PBDB-T-S:SCPDT-PDI4-Se absorber layers under illumination of AM 1.5G at 100 mW cm−2. | |
Table 2 Performance of the optimized OSC devices based on the active layer of PBDB-T-S:acceptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) under illumination of AM 1.5G at 100 mW cm−2
1, w/w) under illumination of AM 1.5G at 100 mW cm−2
| Active layer |
V
oc (V) |
J
sc (mA) |
FF (%) |
PCEa (%) |
μ
h (cm2 V−1 s−1) |
μ
e (cm2 V−1 s−1) |
μ
h/μe |
|
The first value in each is the best PCE, while the one in parentheses is the average PCE collected from 10 independent devices.
|
| PBDB-T-S:SCPDT-PDI4-S |
1.00 |
11.72 |
59.27 |
6.95 (6.90) |
1.960 × 10−5 |
1.751 × 10−5 |
1.12 |
| PBDB-T-S:SCPDT-PDI4-Se |
0.97 |
8.70 |
50.55 |
4.26 (4.16) |
1.779 × 10−5 |
1.097 × 10−5 |
1.62 |
The incident photon to current efficiency (IPCE) spectra of the SCPDT-PDI4-S device and SCPDT-PDI4-Se device are displayed in Fig. 4b. Both the PBDB-T-S:SCPDT-PDI4-S and PBDB-T-S:SCPDT-PDI4-Se blend films exhibit a broad spectral response from 320 to 720 nm. The optimal SCPDT-PDI4-S device exhibits a stronger photo response in the wavelength range of 400–670 nm with a maximum quantum efficiency of 62%. The Jsc values integrated from the IPCE data are 11.34 and 8.33 mA cm−2 for the SCPDT-PDI4-S and SCPDT-PDI4-Se devices, respectively, which are consistent with the values obtained from the J–V measurements (less than 5% mismatch).
To further account for the superior performance of the SCPDT-PDI4-S device, the morphologies and the charge carrier mobilities of the OSC active layers were investigated. The surface morphology was characterized by atomic force microscopy (AFM) and transmission electron microscopy (TEM). It can be seen in Fig. 5a–d that the as-cast blend films of PBDB-T-S:SCPDT-PDI4-S and PBDB-T-S:SCPDT-PDI4-Se display smooth and uniform morphologies. The root mean square (RMS) surface roughness values are 2.443 nm and 2.705 nm, respectively. No distinct molecular aggregations between the donor PBDB-T-S and the two acceptors take place. Compared with the PBDB-T-S:SCPDT-PDI4-Se film, the PBDB-T-S:SCPDT-PDI4-S film exhibits some improvement in the RMS surface roughness values, which could be beneficial to the enhancement of FF and Jsc in the device.23 The AFM image of the PBDB-T-S:SCPDT-PDI4-S film also seems to exhibit a certain nanofibrillar structure, which facilitates charge collection and transport. The TEM images demonstrate that the domain size of the PBDB-T-S:SCPDT-PDI4-S film is smaller than that of the PBDB-T-S:SCPDT-PDI4-Se film (Fig. 5e and f), indicating more suppressed aggregation, which will facilitate exciton dissociation and hence improve the FF and Jsc. As expected, SCPDT-PDI4-S, with the more twisted molecular configuration, gives rise to better surface morphology of the blend film.
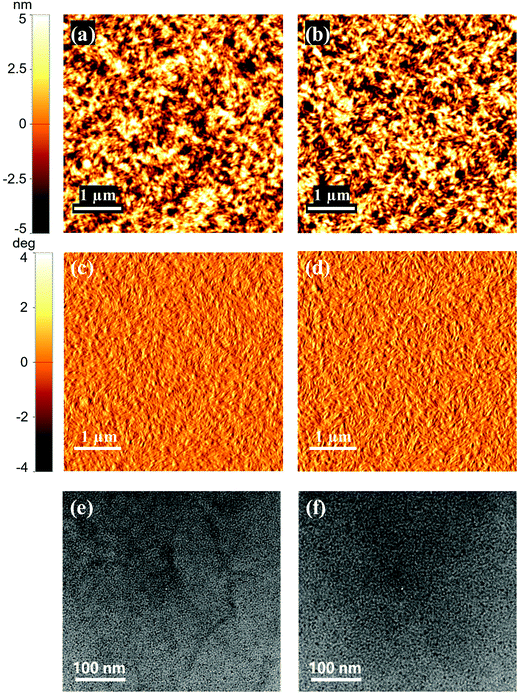 |
| | Fig. 5 Morphology images of PBDB-T-S:SCPDT-PDI4-S (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w; left) and PBDB-T-S:SCPDT-PDI4-Se (1 1, w/w; left) and PBDB-T-S:SCPDT-PDI4-Se (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w; right) blend films: (a) and (b), AFM height; (c) and (d), AFM phase; (e) and (f), TEM images. 1, w/w; right) blend films: (a) and (b), AFM height; (c) and (d), AFM phase; (e) and (f), TEM images. | |
Charge transport characteristics play an important role in device performance; hence we investigated the charge carrier mobility of the donor–acceptor blend film with the space-charge-limited current (SCLC) method, as shown in Fig. 6. Hole-only and electron-only devices were fabricated with the structures ITO/PETDOT:PSS/active layer/MoO3/Al and ITO/ZnO/active layer/PDIN/Al, respectively. The hole mobilities (μh) of PBDB-T-S:SCPDT-PDI4-S and PBDB-T-S:SCPDT-PDI4-Se active layers were calculated to be 1.960 × 10−5 and 1.779 × 10−5 cm2 V−1 s−1, respectively, while the electron mobilities (μe) are 1.751 × 10−5 and 1.097 × 10−5 cm2 V−1 s−1, respectively. Therefore, PBDB-T-S:SCPDT-PDI4-S shows higher μh and μe values as well as more balanced μh/μe values compared with PBDB-T-S:SCPDT-PDI4-Se (see Table 2), which also reasonably accounts for the higher Jsc and FF values of SCPDT-PDI4-S device.
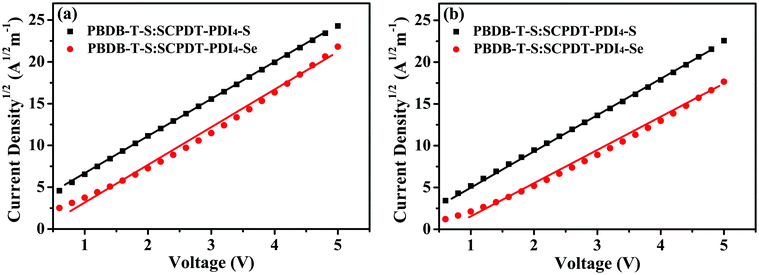 |
| | Fig. 6 Dark current density–voltage characteristics for (a) hole-only devices and (b) electron-only devices with optimized PBDB-T-S:SCPDT-PDI4-S and PBDB-T-S:SCPDT-PDI4-Se active layers. | |
Conclusions
In summary, we designed and synthesized two new non-fullerene SMAs of SCPDT-PDI4-S and SCPDT-PDI4-Se with SCPDT as the spiro core and PDI-S and PDI-Se as the peripheral groups. In comparison with SCPDT-PDI4-Se, SCPDT-PDI4-S exhibits the more suitable absorption properties and more twisted molecular configuration, as well as higher carrier mobility in the blend film. The BHJ OSCs based on the PBDB-T-S:SCPDT-PDI4-S active layer achieve a high PCE of 6.95%, with Voc of 1.00 V, Jsc of 11.72 mA cm−2 and FF of 59.27%, showing a better performance than PTB7-Th:SCPDT-PDI4-based devices without additive, while the OSCs based on the PBDB-T-S:SCPDT-PDI4-Se active layer show lower performance. The good photovoltaic property of SCPDT-PDI4-S makes it a suitable candidate for non-fullerene acceptors in OSCs. The design strategy of SMAs with suitable spiro core and S-annulated PDI end groups is a simple and efficient method to achieve high performance OSCs. It can be expected that with further optimization of energy levels and optical absorption, the corresponding devices can achieve better efficiency in the future.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of Jiangsu Province (Grant No. BK20191358, BK20181373 and BK20170985) and the National Natural Science Foundation of China (Grant No. 11504168). We are also grateful to the High Performance Computing Center of Nanjing Tech University for the supporting computational resources.
Notes and references
- J. Hou, O. Inganäs, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119 CrossRef CAS PubMed.
- G. Li, W.-H. Chang and Y. Yang, Nat. Rev. Mater., 2017, 2, 17043 CrossRef CAS.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2015, 49, 175 CrossRef PubMed.
- L. Lu, M. A. Kelly, W. You and L. Yu, Nat. Photonics, 2015, 9, 491 CrossRef CAS.
- C. B. Nielsen, S. Holliday, H.-Y. Chen, S. J. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803 CrossRef CAS PubMed.
- P. Sonar, J. P. F. Lim and K. L. Chan, Energy Environ. Sci., 2011, 4, 1558 RSC.
- V. Vohra, K. Kawashima, T. Kakara, T. Koganezawa, I. Osaka, K. Takimiya and H. Murata, Nat. Photonics, 2015, 9, 403 CrossRef CAS.
- C. Yan, S. Barlow, Z. Wang, H. Yan, A. K.-Y. Jen, S. R. Marder and X. Zhan, Nat. Rev., 2018, 3, 18003 CAS.
- G. Zhang, J. Zhao, P. C. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447 CrossRef CAS PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666 CrossRef CAS PubMed.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- S. Holliday, R. S. Ashraf, C. B. Nielsen, M. Kirkus, J. A. Röhr, C.-H. Tan, E. C. Fregoso, A. C. Knall, J. R. Durrant, J. Nelson and I. McCulloch, J. Am. Chem. Soc., 2015, 137, 898 CrossRef CAS PubMed.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- Z. Luo, H. Bin, T. Liu, Z.-G. Zhang, Y. Yang, C. Zhong, B. Qiu, G. Li, W. Gao, D. Xie, K. Wu, Y. Sun, F. Liu, Y. Li and C. Yang, Adv. Mater., 2018, 30, 1706124 CrossRef PubMed.
- J. Liu, S. Chen, D. Qian, B. Gautam, G. Yang, J. Zhao, J. Bergqvist, F. Zhang, W. Ma, H. Ade, O. Inganäs, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 16089 CrossRef CAS.
- D. Meng, D. Sun, C. Zhong, T. Liu, B. Fan, L. Huo, Y. Li, W. Jiang, H. Choi, T. Kim, J. Y. Kim, Y. Sun, Z. Wang and A. J. Heeger, J. Am. Chem. Soc., 2015, 138, 375 CrossRef PubMed.
- Y. Zhong, M. T. Trinh, R. Chen, G. E. Purdum, P. P. Khlyabich, M. Sezen, S. Oh, H. Zhu, B. Fowler, B. Zhang, W. Wang, C. Nam, M. Y. Sfeir, C. T. Black, M. L. Steigerwald, Y. Loo, F. Ng, X. Zhu and C. Nuckolls, Nat. Commun., 2015, 6, 8242 CrossRef CAS PubMed.
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140 CrossRef CAS.
- S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423 CrossRef CAS PubMed.
- Z. Luo, T. Liu, Z. Chen, Y. Xiao, G. Zhang, L. Huo, C. Zhong, X. Lu, H. Yan, Y. Sun and C. Yang, Adv. Sci., 2019, 6, 1802065 CrossRef PubMed.
- D. Meng, H. Fu, C. Xiao, X. Meng, T. Winands, W. Ma, W. Wei, B. Fan, L. Huo, N. L. Doltsinis, Y. Li, Y. Sun and Z. Wang, J. Am. Chem. Soc., 2016, 138, 10184 CrossRef CAS PubMed.
- J. Zhang, Y. Li, J. Huang, H. Hu, G. Zhang, T. Ma, P. C. Chow, H. Ade, D. Pan and H. Yan, J. Am. Chem. Soc., 2017, 139, 16092 CrossRef CAS PubMed.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Adv. Mater., 2017, 29, 1700144 CrossRef PubMed.
- B. Fan, D. Zhang, M. Li, W. Zhong, Z. Zeng, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2019, 62, 746 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, T. Zhang, Y. Wang, L. Hong, K. Xian, B. Xu, S. Zhang, J. Peng, Z. Wei, F. Gao and J. Hou, Nat. Commun., 2019, 10, 2515 CrossRef PubMed.
- G. Li, S. Wang, T. Liu, P. Hao, Z. Liu, F. Li, L.-M. Yang, Y. Zhang, D. Li, S. Yang, J. Zhao, J. Li, H. Yan and B. Tang, J. Mater. Chem. C, 2018, 6, 12601 RSC.
- Y. Lin, B. Adilbekova, Y. Firdaus, E. Yengel, H. Faber, M. Sajjad, X. Zheng, E. Yarali, A. Seitkhan, O. M. Bakr, A. El-Labban, U. Schwingenschlögl, V. Tung, I. McCulloch, F. Laquai and T. D. Anthopoulos, Adv. Mater., 2019, 31, 1902965 CrossRef CAS PubMed.
- Y. Lin, Y. Firdaus, M. I. Nugraha, F. Liu, S. Karuthedath, A.-H. Emwas, W. Zhang, A. Seitkhan, M. Neophytou, H. Faber, E. Yengel, I. McCulloch, L. Tsetseris, F. Laquai and T. D. Anthopoulos, Adv. Sci., 2020, 1903419 CrossRef PubMed.
- L. Zhan, S. Li, T. Lau, Y. Cui, X. Lu, M. Shi, C. Li, H. Li, J. Hou and H. Chen, Energy Environ. Sci., 2020, 13, 635 RSC.
- Y. Duan, X. Xu, H. Yan, W. Wu, Z. Li and Q. Peng, Adv. Mater., 2017, 29, 1605115 CrossRef PubMed.
- T. Liu, D. Meng, Y. Cai, X. Sun, Y. Li, L. Huo, F. Liu, Z. Wang, T. P. Russell and Y. Sun, Adv. Sci., 2016, 3, 1600117 CrossRef PubMed.
- H. Sun, X. Song, J. Xie, P. Sun, P. Gu, C. Liu, F. Chen, Q. Zhang, Z.-K. Chen and W. Huang, ACS Appl. Mater. Interfaces, 2017, 9, 29924 CrossRef CAS PubMed.
- Z. Liu, Y. Wu, Q. Zhang and X. Gao, J. Mater. Chem. A, 2016, 4, 17604 RSC.
- Y. Lin and X. Zhan, Adv. Energy Mater., 2015, 5, 1501063 CrossRef.
- W. Fan, N. Liang, D. Meng, J. Feng, Y. Li, J. Hou and Z. Wang, Chem. Commun., 2016, 52, 11500 RSC.
- Z. Luo, T. Liu, W. Cheng, K. Wu, D. Xie, L. Huo, Y. Sun and C. Yang, J. Mater. Chem. C, 2018, 6, 1136 RSC.
- D. Sun, D. Meng, Y. Cai, B. Fan, Y. Li, W. Jiang, L. Huo, Y. Sun and Z. Wang, J. Am. Chem. Soc., 2015, 137, 11156 CrossRef CAS PubMed.
- J. Cann, S. Dayneko, J.-P. Sun, A. D. Hendsbee, I. G. Hill and G. C. Welch, J. Mater. Chem. C, 2017, 5, 2074 RSC.
- J. Londenberg, T. P. Saragi, I. Suske and J. Salbeck, Adv. Mater., 2007, 19, 4049 CrossRef CAS.
- H. Sun, P. Sun, C. Zhang, Y. Yang, X. Gao, F. Chen, Z. Xu, Z.-K. Chen and W. Huang, Chem. – Asian J., 2017, 12, 721 CrossRef CAS PubMed.
- M. Wang, H. Wang, T. Yokoyama, X. Liu, Y. Huang, Y. Zhang, T.-Q. Nguyen, S. Aramaki and G. C. Bazan, J. Am. Chem. Soc., 2014, 136, 12576 CrossRef CAS PubMed.
-
J. W. Ponder, TINKER: Software Tools for Molecular Design, Washington University School of Medicine, Saint Louis, MO, 2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tc00341g |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a and
Wei
Huang
*a and
Wei
Huang
 ab
ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) are characterized, which confirm a full harvest of light in the visible spectrum, as shown in Fig. S1 (ESI†). As expected, the PBDB-T-S:SCPDT-PDI4-S blend shows superior absorbance compared with PBDB-T-S:SCPDT-PDI4-Se one.
1, w/w) are characterized, which confirm a full harvest of light in the visible spectrum, as shown in Fig. S1 (ESI†). As expected, the PBDB-T-S:SCPDT-PDI4-S blend shows superior absorbance compared with PBDB-T-S:SCPDT-PDI4-Se one.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a total concentration of 20 mg mL−1 in dichlorobenzene. The current density–voltage (J–V) characteristics of the devices are shown in Fig. 4a and the parameters are summarized in Table 2. The PBDB-T-S:SCPDT-PDI4-S-based device (SCPDT-PDI4-S device) achieves a high PCE of 6.95% along with a high Voc of 1.00 V, Jsc of 11.72 mA cm−2 and fill factor (FF) of 59.27%, while the PBDB-T-S:SCPDT-PDI4-Se-based device (SCPDT-PDI4-Se device) has a PCE of 4.26%, along with a Voc of 0.97 V, Jsc of 8.70 mA cm−2 and FF of 50.55%. It can be easily seen the SCPDT-PDI4-S device shows the much higher Jsc, which can be ascribed to the more complementary absorbance of SCPDT-PDI4-S with the donor molecule. In addition, the larger FF of the SCPDT-PDI4-S device is believed to arise from the more favorable morphology of PBDB-T-S:SCPDT-PDI4-S film compared with that of PBDB-T-S:SCPDT-PDI4-Se film, which would reduce the impedance at the interface. Note that the SCPDT-PDI4-S device also shows a higher efficiency than the SCPDT-PDI4-based device with the different polymer donor PTB7-Th (PCE = 6.31%, without additive), as reported in the literature.42 In our study, the donor PTB7-Th paired with SCPDT-PDI4-S and SCPDT-PDI4-Se acceptors was also tried but led to a relatively low performance.
1, with a total concentration of 20 mg mL−1 in dichlorobenzene. The current density–voltage (J–V) characteristics of the devices are shown in Fig. 4a and the parameters are summarized in Table 2. The PBDB-T-S:SCPDT-PDI4-S-based device (SCPDT-PDI4-S device) achieves a high PCE of 6.95% along with a high Voc of 1.00 V, Jsc of 11.72 mA cm−2 and fill factor (FF) of 59.27%, while the PBDB-T-S:SCPDT-PDI4-Se-based device (SCPDT-PDI4-Se device) has a PCE of 4.26%, along with a Voc of 0.97 V, Jsc of 8.70 mA cm−2 and FF of 50.55%. It can be easily seen the SCPDT-PDI4-S device shows the much higher Jsc, which can be ascribed to the more complementary absorbance of SCPDT-PDI4-S with the donor molecule. In addition, the larger FF of the SCPDT-PDI4-S device is believed to arise from the more favorable morphology of PBDB-T-S:SCPDT-PDI4-S film compared with that of PBDB-T-S:SCPDT-PDI4-Se film, which would reduce the impedance at the interface. Note that the SCPDT-PDI4-S device also shows a higher efficiency than the SCPDT-PDI4-based device with the different polymer donor PTB7-Th (PCE = 6.31%, without additive), as reported in the literature.42 In our study, the donor PTB7-Th paired with SCPDT-PDI4-S and SCPDT-PDI4-Se acceptors was also tried but led to a relatively low performance.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) under illumination of AM 1.5G at 100 mW cm−2
1, w/w) under illumination of AM 1.5G at 100 mW cm−2

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w; left) and PBDB-T-S:SCPDT-PDI4-Se (1
1, w/w; left) and PBDB-T-S:SCPDT-PDI4-Se (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w; right) blend films: (a) and (b), AFM height; (c) and (d), AFM phase; (e) and (f), TEM images.
1, w/w; right) blend films: (a) and (b), AFM height; (c) and (d), AFM phase; (e) and (f), TEM images.


