Fast transformation of a rare-earth doped luminescent sub-microcrystal via plasmonic nanoislands†
Abstract
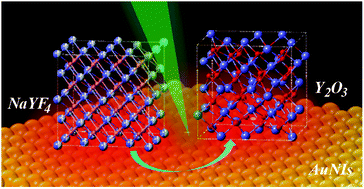
An efficient and fast transformation scheme for the matrix crystal of rare-earth doped luminescent micro-nanomaterials is developed by using plasmonic gold/silver nanoislands. The transformation is realized through an oxidation reaction from a polycrystalline sub-microcrystal to a single crystal, accompanied by the optimization of the crystal structure and a significant increase in luminescence. The crystal transformation can be achieved in tens of milliseconds, and the rate is controlled not only by the laser illumination power and wavelength, but also by the size and nanogap of nanoislands. Particularly, single crystal transformation is also achieved even at very low temperature, which provides a new way to obtain single crystal materials in a harsh environment. Moreover, the crystal transformation efficiency of the gold plasmonic islands is very stable in air over at least three months. This plasmon driven crystal transformation rapidly provides highly crystalline nanomaterials, which breaks the dependence of high temperature, long period and high energy consumption in the traditional annealing treatment.


 Please wait while we load your content...
Please wait while we load your content...