Color manipulation of Bi3+-activated CaZnOS under stress with ultra-high efficiency and low threshold for anticounterfeiting applications†
Abstract
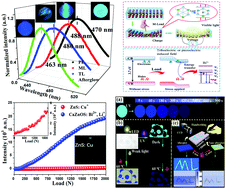
We report blue to green emission mechanoluminescence (ML) in CaZnOS:Bi3+,Li+with about 50 times greater ML intensity than that of the strong ML material ZnS:Cu+. The ML spectrum showed an intense emission band peaking at 485 nm and a red-shift of about 9 nm on increasing the stress or friction. In addition, the preferable threshold of CaZnOS:Bi3+,Li+ was below 1 N due to its excellent strain sensitivity. The depths of the traps responsible for ML were estimated to be in the range of 0.68–1.2 eV. The as-prepared CaZnOS:Bi3+,Li+ phosphors were successfully used for the fabrication of homemade ink and flexible thin films, which could be further used for anticounterfeiting and stress mapping. These findings reveal its great potential for application in an advanced anticounterfeiting technology and visualization of stress distribution for damage diagnosis due to its ultra-high efficiency, low threshold and color tunability.


 Please wait while we load your content...
Please wait while we load your content...