Optimization of processing solvent and film morphology to achieve efficient non-fullerene polymer solar cells processed in air†
Kang
An
a,
Wenkai
Zhong
a,
Lei
Ying
 *ab,
Peng
Zhu
a,
Baobing
Fan
ab,
Zhenye
Li
a,
Ning
Li
*ab,
Peng
Zhu
a,
Baobing
Fan
ab,
Zhenye
Li
a,
Ning
Li
 *c,
Fei
Huang
*c,
Fei
Huang
 *ab and
Yong
Cao
a
*ab and
Yong
Cao
a
aInstitute of Polymer Optoelectronic Materials and Devices, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, 510640, P. R. China. E-mail: msleiying@scut.edu.cn; msfhuang@scut.edu.cn
bSouth China Institute of Collaborative Innovation, Dongguan 523808, China
cInstitute of Materials for Electronics and Energy Technology (i-MEET), Friedrich-Alexander-Universität Erlangen-Nürnberg, 91058, Erlangen, Germany. E-mail: ning.li@fau.de
First published on 25th November 2019
Abstract
Polymer solar cells (PSCs) with remarkable power conversion efficiency and processability have been widely reported; however, most devices are processed using halogenated solvents under an inert atmosphere and require post-treatment to achieve optimal film morphology. In this manuscript, we developed efficient PSCs by combining a wide-bandgap conjugated polymer P2F-EHp and non-fullerene acceptors of IT-4F and IT-4Cl, which can be processed with non-chlorinated toluene:o-xylene co-solvent. It is interesting to note that a device based on IT-4Cl presented impressive photovoltaic performance with a power conversion efficiency of about 12%, which does not require post-treatment of solvent vapor annealing. The detailed investigation of film morphology by grazing incidence X-ray scattering and resonant soft X-ray scattering demonstrated that the co-solvent appeared to assist the manipulation of crystal coherent lengths and effectively decrease the phase separation of the corresponding blend films. Of particular importance is that this material system is compatible with the low-cost blade-coating technique using toluene:o-xylene co-solvent and can be processed under ambient conditions without post-treatment. A remarkable power conversion efficiency of 10.1% was achieved by blade-coating the P2F-EHp:IT-4F:IT-4Cl in air, which is slightly higher than that of 9.94% obtained from the spin-coating device processed in nitrogen. The results indicated that this material system is a promising candidate for constructing efficient PSCs toward practical applications.
1. Introduction
In the past two decades, polymer solar cells (PSCs) have attracted extensive attention because of their advantages including low-cost, light weight, short energy payback time, and compatibility for large-area flexible devices.1–5 Very recently, the power conversion efficiency (PCE) of single junction PSCs has been boosted to over 16% by virtue of the emergence of new light-harvesting materials, including both high performance conjugated polymer donors and non-fullerene acceptors (NFAs).6–12 In general, the light-harvesting layer of so far reported high-performance PSCs is typically fabricated by using a certain amount of halogenated solvent additive,13–16 and the processing procedure is usually carried out in an inert atmosphere, which requires post-treatment such as thermal annealing and/or solvent vapor annealing (SVA) to achieve optimal morphology.17,18 An additional issue that arouses particular interests is the processing solvents, which are typically chlorinated, such as chlorobenzene, 1,2-dichlorobenzene, chloroform, and so forth. In this regard, non-chlorinated solvents that are less toxic than the chlorinated counterparts are highly appreciated for developing high performance PSCs toward mass production.Due to the priority of controlling film quality, spin-coating is widely used for fabricating small-area devices, yet the spin-coating technique is not well-matched with the high-throughput printing procedure toward practical applications.19–21 Additionally, even though a wide range of halogenated-free solvents and additives have been recently employed in PSC fabrication,22–25 their applications in relevant compatible printing techniques, such as blade-coating or slot-die coating, still lag behind for constructing PSCs. Among these widely used processing techniques, blade-coating is compatible with the low-cost, high-throughput roll-to-roll manufacturing technique.26 The film morphology of blade-coated films can be controlled by adjusting the blading speed, the substrate temperature, and the gap between the blade and substrate, which shows great potential in processing solar cell devices.27,28
Recently, we developed a non-fullerene PSC based on a wide-bandgap polymer donor P2F-EHp and an NFA of IT-4F, providing an impressively high power conversion efficiency (PCE) of over 12% with an active layer area of about 1 cm2,29 and the performance can be further enhanced by optimizing film morphology.30 This progress urges us to dedicate more effort to addressing the goals in PSCs, that is, simplifying the post-treatment procedures, exchanging the chlorinated processing solvents to non-chlorinated counterparts, using the blade-coating method to replace the spin-coating technique, and so forth. Therefore, in this work, we fabricated non-fullerene PSCs based on P2F-EHp and NFA with different terminal groups, among which devices based on P2F-EHp and 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-dichloro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene (IT-4Cl) exhibited less dependence of PCE on the solvent vapor annealing procedure than the counterpart device based on P2F-EHp:IT-4F.31 It is interesting to note that the non-chlorinated solvents of toluene and o-xylene with optimal volume ratio can be used to replace chloroform, which presents pronounced effects on the nanostructure of the blend film, and thus overall photovoltaic performances. Of particular importance is that the developed material system presented a promising PCE over 10% by blade-coating under ambient conditions, indicating great promise toward practical applications.
2. Results and discussion
2.1 Optimization of photovoltaic performances
The molecular structures of P2F-EHp, IT-4F and IT-4Cl are shown in Fig. 1a. The absorption of P2F-EHp is primarily located in the range of 380–650 nm, which is complementary to the absorption profiles of IT-4F or IT-4Cl that have strong absorption in the low energy band from 500 to about 800 nm (Fig. 1b). It is worth noting that the absorption profile of IT-4Cl slightly red-shifted compared to that of IT-4F, which indicates more favourable light-harvesting characteristics of the former.32 The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels for P2F-EHp are −5.38 eV and −3.06 eV, respectively, which is compatible with those of −5.64/−4.05 eV for IT-4F and −5.75/−4.27 eV for IT-4Cl.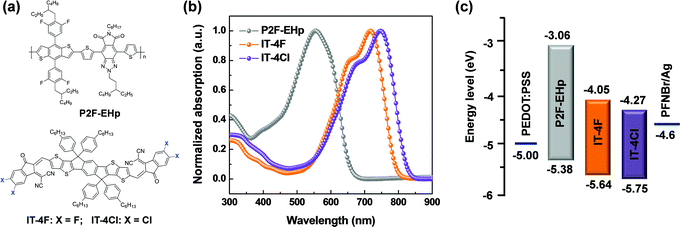 | ||
| Fig. 1 (a) Molecular structures of materials. (b) Absorption spectra of P2F-EHp, IT-4F and IT-4Cl as thin films. (c) Energy level diagram of the devices. | ||
To evaluate the photovoltaic performances of devices based on P2F-EHp:IT-4F and P2F-EHp:IT-4Cl, we fabricated devices with a conventional structure of indium tin oxide (ITO)/poly(3,4-ethylenedioxythiophene):poly(styrenesulphonate) (PEDOT:PSS)/P2F-EHp:NFA/PFN-Br/Ag. Here PFN-Br with a thickness of about 5 nm is used as the cathode interfacial layer to facilitate electron collection, which is spin-coated on the top of the prefabricated P2F-EHp:NFA layer. The device based on the as-cast P2F-EHp:IT-4F film exhibits a PCE of 10.92%, with an open-circuit voltage (VOC) of 0.92 V, a short-circuit current density (JSC) of 18.04 mA cm−2, and a fill factor (FF) of 65.43%. It has been reported that the solvent vapor annealing (SVA) treatment of the light-harvesting layer can allow for the rearrangement of the polymer main chain to achieve optimal film morphology.33 For the P2F-EHp:IT-4F bulk-heterojunction film, upon SVA treatment by using chloroform for 60 s, the PCE was improved to 12.64% by virtue of the enhanced JSC of 19.56 mA cm−2 and FF of 71.40% (Fig. 2a). The enhanced JSC is consistent with the slightly extended absorption profile of the P2F-EHp:IT-4F blend film and can be further confirmed by the enhanced and extended external quantum efficiency (EQE) spectrum of the device upon SVA treatment (Fig. S1, ESI†). In contrast, the device based on P2F-EHp:IT-4Cl presented a similar PCE of about 12%, yet the PCE is insensitive to the SVA treatment (Fig. 2d). Detailed photovoltaic parameters are summarized in Table 1.
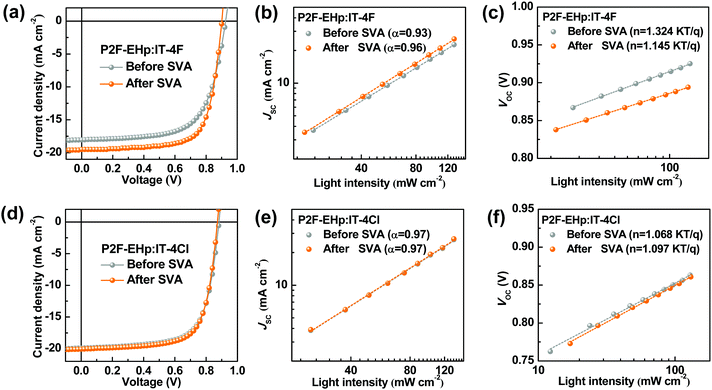 | ||
| Fig. 2 (a and d) J–V characteristics, (b and e) JSC as a function of light intensity, and (c and f) VOC as a function of light intensity of devices based on P2F-EHp:IT-4F and P2F-EHp:IT-4Cl. | ||
| Acceptora | V OC (V) | J SC (mA cm−2) | J SC,EQE (mA cm−2) | FF (%) | PCE (PCEavgd) (%) |
|---|---|---|---|---|---|
| a Devices with active area of 0.04 cm2. b Solvent vapor annealing for 60 s. c J SC,EQE represents the integrated current density obtained from EQE spectra. d Statistical data obtained from 10 devices. | |||||
| IT-4F | 0.92 | 18.04 | 17.23 | 65.43 | 10.92 (10.82 ± 0.10) |
| IT-4Fb | 0.90 | 19.56 | 18.55 | 71.40 | 12.64 (12.54 ± 0.10) |
| IT-4Cl | 0.88 | 19.90 | 19.74 | 69.14 | 12.14 (12.02 ± 0.12) |
| IT-4Clb | 0.87 | 20.06 | 19.90 | 70.69 | 12.37 (12.24 ± 0.13) |
From the characteristics of JSC as a function of light intensity (Ilight) as shown in Fig. 2b, the power-law exponent (α) of the JSC ∝ (Ilight)α is determined to be 0.93 and 0.96 for devices based on P2F-EHp:IT-4F before and after SVA treatment, respectively. A slightly higher α of the device with SVA treatment implies suppressed bimolecular recombination. In addition, from the characteristics of the VOCversus light intensity, one can note that the slopes for the P2F-EHp:IT-4F device before and after SVA treatment are 1.32kT/q and 1.14kT/q, respectively, where k is the Boltzmann constant, T is Kelvin temperature, and q is unit charge. The lower slope of the device upon SVA treatment clearly indicates the reduced Shockley–Read–Hall recombination.34 Moreover, the P2F-EHp:IT-4F device with SVA treatment presents slightly higher charge dissociation probability P(E,T) of 96.4% than that of 94.4% at short-circuit conditions without SVA treatment, which also suggests the higher exciton dissociation of the former. It is also worth noting that the SVA treatment leads to rougher film morphology of the P2F-EHp:IT-4F blend film, with the root-mean-square roughness increased from 1.24 nm for the pristine film to 4.44 nm after SVA treatment (Fig. S2, ESI†). In contrast, devices based on P2F-EHp:IT-4Cl before and after SVA treatment present nearly identical power-law exponent (α = 0.97), similar slope of VOC-light intensity and P(E,T) values (Fig. S1, ESI†), and similar surface morphology (Fig. S2, ESI†), which agree with the insensitive photovoltaic performances upon SVA treatment for the P2F-EHp:IT-4Cl system.
2.2 Devices processed with non-chlorinated solvent
From the perspective of practical applications, the chlorinated solvents, such as chloroform and chlorobenzene, are undesirable. Thus, here we utilized two high boiling point (b.p.) non-chlorinated solvents, toluene (b.p. of 110.6 °C) and o-xylene (b.p. of 144.4 °C), to process these devices instead of chloroform. Our concerns focus on the P2F-EHp:IT-4Cl based device, as it presents higher photovoltaic performance than the P2F-EHp:IT-4F counterpart. It is interesting to note that the P2F-EHp:IT-4Cl blend films processed with toluene, o-xylene and the toluenen:o-xylene co-solvent presented slightly different absorption profiles (Fig. S4, ESI†), which might be correlated to the difference in aggregation behaviors. The J–V characteristics and EQE spectra of these devices are shown in Fig. 3. Devices processed with toluene and o-xylene exhibit moderate photovoltaic performances with PCEs of 7.81% (VOC = 0.82 V, JSC = 17.58 mA cm−2, FF = 53.83%) and 8.26% (VOC = 0.86 V, JSC = 17.66 mA cm−2, FF = 54.03%), respectively, both of which are much lower than that of the device processed with chloroform. However, it is interesting to note that the device processed with toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o-xylene co-solvent (volume ratio of 1
o-xylene co-solvent (volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) presents a much higher PCE of 9.51% (VOC = 0.89 V, JSC = 17.59 mA cm−2, FF = 60.00%).
2) presents a much higher PCE of 9.51% (VOC = 0.89 V, JSC = 17.59 mA cm−2, FF = 60.00%).
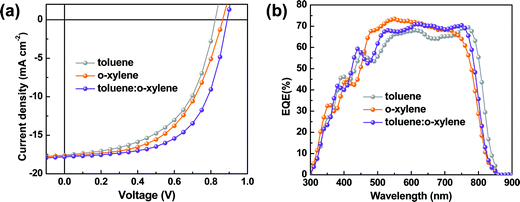 | ||
Fig. 3 (a) J–V characteristics for the P2F-EHp:IT-4Cl devices processed with toluene, o-xylene and toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o-xylene (1 o-xylene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). (b) EQE spectra for the corresponding devices. 2). (b) EQE spectra for the corresponding devices. | ||
The accuracy of the obtained JSC values from the current density as a function of voltage characteristics are consistent with those integrated from the external quantum efficiency (EQE) curves. From the EQE spectra, one notes that the device processed with toluene exhibited slightly red-shifted characteristics compared to those processed with o-xylene or the toluene:o-xylene co-solvent. Such a red-shift is consistent with the absorption spectra of the P2F-EHp:IT-4Cl blend film (Fig. S4, ESI†), which might be correlated to the different aggregation or morphology of these films.
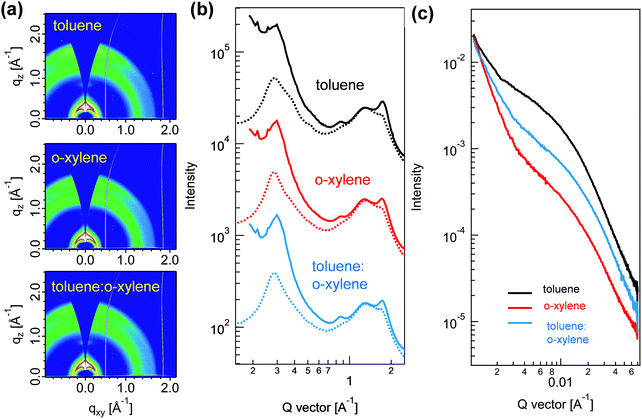
To disclose the obviously enhanced PCE of the device processed with toluene:o-xylene co-solvent, we initially compared the nanoscale structure of the P2F-EHp:IT-4Cl blend films processed with different solvents by using grazing incidence wide-angle X-ray scattering (GIWAXS). It is noted that the blend films processed from toluene, o-xylene and the toluene:o-xylene mixture presented similar scattering patterns, all of which showed a strong (010) peak at the q value of 1.74 Å−1 in the out-of-plane direction (Fig. 4a and b). The interlayer lamellar stacking (100) peak at the q value of about 0.3 Å−1 in the in-plane (IP) direction is the combination of the interferences that are created by both P2F-EHp and IT-4Cl alkyl–alkyl stacking, which can be easily separated by curve fitting (Fig. S6, ESI†). The film processed with toluene presented a relatively high crystal coherent length (CCL) value of 7.3 nm for P2F-EHp, which increased to 8.9 nm for the film processed with o-xylene, and to 8.1 nm for the film processed with toluene:o-xylene co-solvent. In contrast, the CCL values of IT-4Cl slightly decreased from 4.5 nm for the toluene processed film to 3.3 nm for the toluene:o-xylene co-solvent processed film. These findings suggested that the co-solvent appeared to assist the manipulation of the CCLs of both the components in the blend films. To quantify the crystallinity changes of blends processed using different solvents, we estimated relative degree of crystallinity (rDoC) of each thin film from the pole figures, which were depicted by plotting the (010) peak intensity as a function of azimuthal angle (Fig. S7, ESI†).35 The film prepared by o-xylene was found to have the highest relative crystallinity. The toluene processed blend also showed high crystallinity as the rDoC was only decreased by 6%. When the processing solvent was switched to toluene:o-xylene, the rDoC was further reduced by 12%. Although it is generally accepted that higher crystallinity suggests more efficient charge transport inside the thin film, the rDoC presented here is an estimation of the overall crystallinity for both P2F-EHp and IT-4Cl. Thus, it is highly plausible that the higher device FF for the toluene:o-xylene co-solvent might originate from the improved charge transport balance.36
The effects of processing solvent on the morphology of these blend films can also be disclosed by resonant soft X-ray scattering (RSoXS). The measurement was carried out at a beam energy of 284.2 eV, with the corresponding characteristics shown in Fig. 4c. All blends showed similar scattering profiles with broad humps within the probed q range. The statistic center-to-center domain distances are 39.3 nm (q = 0.016 Å−1), 27.3 nm (q = 0.023 Å−1), and 28.5 nm (q = 0.022 Å−1) for toluene, o-xylene, and toluene:o-xylene processed blend films, respectively. The obviously decreased center-to-center domain distances indicated that the incorporation of o-xylene as the co-solvent can effectively decrease the phase separation of these blend films. This observation is consistent with those found in the transmission electron microscopy images (Fig. S8, ESI†).
2.3 Blade-coating devices processed with non-chlorinated solvents
Having established the priority of processing this P2F-EHp:IT-4Cl binary blend system by using non-chlorinated toluene:o-xylene co-solvent, we then constructed ternary solar cells consisting of P2F-EHp as the donor and IT-4Cl:IT-4F mixture as the electron acceptor, since the very similar molecular structure of IT-4F and IT-4Cl may lead to a more favorable film morphology. For the device processed by spin-coating under an inert N2 atmosphere, the optimal ratio of the ternary blends P2F-EHp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IT-4F
IT-4F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IT-4Cl (weight ratio of 1
IT-4Cl (weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8) presented a PCE of 9.94% (VOC = 0.91 V, JSC = 17.14 mA cm−2, FF = 63.86%), which is slightly higher than that of the binary device based on P2F-EHp:IT-4Cl. Considering that the electron-acceptors of IT-4F and IT-4Cl have very similar molecular structure, it is highly possible that these two acceptors may form alloy-like structures in the P2F-EHp:IT-4F:IT-4Cl ternary blends.
0.8) presented a PCE of 9.94% (VOC = 0.91 V, JSC = 17.14 mA cm−2, FF = 63.86%), which is slightly higher than that of the binary device based on P2F-EHp:IT-4Cl. Considering that the electron-acceptors of IT-4F and IT-4Cl have very similar molecular structure, it is highly possible that these two acceptors may form alloy-like structures in the P2F-EHp:IT-4F:IT-4Cl ternary blends.
To evaluate the compatibility of this material system toward practical applications, we therefore fabricated devices by blade-coating, which is carried out under ambient conditions using toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o-xylene (1
o-xylene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v
2, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) as the solvent. Impressively, we observed a slightly enhanced PCE of 10.10% (VOC = 0.86 V, JSC = 17.78 mA cm−2, FF = 65.77%), which is, to our knowledge, among the highest PCE values of so far reported PSCs processed by blade-coating under ambient conditions.37–39 The accuracy of the obtained JSC values agreed with those integrated from the EQE spectra (Fig. 5c). These results demonstrated that the P2F-EHp:IT-4F:IT-4Cl, which can be processed with non-chlorinated solvent without post solvent vapor annealing treatment, can be a promising candidate for constructing efficient PSCs toward practical applications (Table 2).
v) as the solvent. Impressively, we observed a slightly enhanced PCE of 10.10% (VOC = 0.86 V, JSC = 17.78 mA cm−2, FF = 65.77%), which is, to our knowledge, among the highest PCE values of so far reported PSCs processed by blade-coating under ambient conditions.37–39 The accuracy of the obtained JSC values agreed with those integrated from the EQE spectra (Fig. 5c). These results demonstrated that the P2F-EHp:IT-4F:IT-4Cl, which can be processed with non-chlorinated solvent without post solvent vapor annealing treatment, can be a promising candidate for constructing efficient PSCs toward practical applications (Table 2).
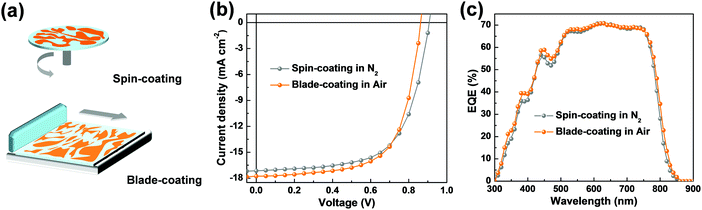 | ||
| Fig. 5 (a) Schematic diagram for the spin-coating and blade-coating technique. (b) J–V characteristics and (c) EQE spectra of devices based on P2F-EHp:IT-4F:IT-4Cl. | ||
| Devicea | V OC (V) | J SC (mA cm−2) | J SC,EQE (mA cm−2) | FF (%) | PCE (PCEavgc) (%) |
|---|---|---|---|---|---|
| a Devices with area of 0.104 cm2. b J SC,EQE represents the integrated current density obtained from EQE spectra. c Statistical data obtained from 10 devices. | |||||
| Spin-coating in N2 | 0.91 | 17.14 | 17.08 | 63.86 | 9.94 (9.83 ± 0.11) |
| Blade-coating in air | 0.86 | 17.78 | 17.50 | 65.77 | 10.10 (9.96 ± 0.05) |
3. Conclusions
In summary, we developed an efficient non-fullerene polymer solar cell by integrating the electron donor of P2F-EHp with the electron-acceptors IT-4F and IT-4Cl. The solvent vapor annealing treatment plays a critical role in the photovoltaic performance of the P2F-EHp:IT-4F based binary device, yet has trivial effects on that of the IT-4Cl based binary device. It is also noted that the P2F-EHp:IT-4Cl based device processed with either toluene or o-xylene exhibited lower power conversion efficiency than that obtained from the device processed with toluene:o-xylene mixture, which can be attributed to the controlled crystal coherent lengths and decreased phase separation of the corresponding blend films, as disclosed by GIWAXS and RSoXS measurements. More importantly, a device based on the ternary blends of P2F-EHp:IT-4F:IT-4Cl exhibited similar photovoltaic performances by spin-coating in an inert nitrogen atmosphere and blade-coating under ambient conditions. To our knowledge, the resulting power conversion of 10.1% that is processed by blade-coating in air is among the highest values of so far reported non-fullerene polymer solar cells, demonstrating the great potential of using the current material system and processing strategy for the construction of efficient polymer solar cells in future mass production.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21822505, 51673069, 21520102006 and 51521002), Program for Science and Technology Development of Dongguan (No. 2019622163009) and the Dongguan Innovative Research Team Program (No. 2018607201002). Portions of this research used the resources of beamline 7.3.3 and 11.0.1.2 at Advanced Light Source, Materials Science Division, The Molecular Foundry, Lawrence Berkeley National Laboratory, which was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.Notes and references
- Z. P. Yu, Z. X. Liu, F. X. Chen, R. Qin, T. K. Lau, J. L. Yin, X. Kong, X. Lu, M. Shi, C. Z. Li and H. Chen, Nat. Commun., 2019, 10, 2152 CrossRef.
- D. Dang, D. Yu and E. Wang, Adv. Mater., 2019, 31, 1807019 CrossRef.
- S. Li, L. Zhan, F. Liu, J. Ren, M. Shi, C. Z. Li, T. P. Russell and H. Chen, Adv. Mater., 2018, 30, 1705208 CrossRef.
- C. Sun, F. Pan, H. Bin, J. Zhang, L. Xue, B. Qiu, Z. Wei, Z. G. Zhang and Y. Li, Nat. Commun., 2018, 9, 743 CrossRef.
- T. Li, S. Dai, Z. Ke, L. Yang, J. Wang, C. Yan, W. Ma and X. Zhan, Adv. Mater., 2018, 30, 1705969 CrossRef.
- F. X. Chen, J. Q. Xu, Z. X. Liu, M. Chen, R. Xia, Y. Yang, T. K. Lau, Y. Zhang, X. Lu, H. L. Yip, A. K. Jen, H. Chen and C. Z. Li, Adv. Mater., 2018, 30, 1803769 CrossRef.
- R. Yu, H. Yao, Z. Chen, J. Xin, L. Hong, Y. Xu, Y. Zu, W. Ma and J. Hou, Adv. Mater., 2019, 31, 1900477 CrossRef.
- Y. Wu, H. Yang, Y. Zou, Y. Dong, J. Yuan, C. Cui and Y. Li, Energy Environ. Sci., 2019, 12, 675 RSC.
- W. Li, M. Chen, J. Cai, E. L. K. Spooner, H. Zhang, R. S. Gurney, D. Liu, Z. Xiao, D. G. Lidzey, L. Ding and T. Wang, Joule, 2019, 3, 819 CrossRef CAS.
- B. Fan, D. Zhang, M. Li, W. Zhong, Z. Zeng, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2019, 62, 746 CrossRef CAS.
- S. Li, L. Ye, W. Zhao, H. Yan, B. Yang, D. Liu, W. Li, H. Ade and J. Hou, J. Am. Chem. Soc., 2018, 140, 7159 CrossRef CAS.
- P. Cheng, G. Li, X. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131 CrossRef CAS.
- R. Sun, J. Guo, C. Sun, T. Wang, Z. Luo, Z. Zhang, X. Jiao, W. Tang, C. Yang, Y. Li and J. Min, Energy Environ. Sci., 2019, 12, 384 RSC.
- J. Sun, X. Ma, Z. Zhang, J. Yu, J. Zhou, X. Yin, L. Yang, R. Geng, R. Zhu, F. Zhang and W. Tang, Adv. Mater., 2018, 30, 1707150 CrossRef.
- G. Zhang, G. Yang, H. Yan, J. H. Kim, H. Ade, W. Wu, X. Xu, Y. Duan and Q. Peng, Adv. Mater., 2017, 29, 1606054 CrossRef PubMed.
- J. Zhu, Z. Ke, Q. Zhang, J. Wang, S. Dai, Y. Wu, Y. Xu, Y. Lin, W. Ma, W. You and X. Zhan, Adv. Mater., 2018, 30, 1704713 CrossRef.
- F. Zhao, C. Wang and X. Zhan, Adv. Energy Mater., 2018, 8, 1703147 CrossRef.
- M. Babics, R.-Z. Liang, K. Wang, F. Cruciani, Z. Kan, M. Wohlfahrt, M.-C. Tang, F. Laquai and P. M. Beaujuge, Chem. Mater., 2018, 30, 789 CrossRef CAS.
- L. Zhang, X. Xu, B. Lin, H. Zhao, T. Li, J. Xin, Z. Bi, G. Qiu, S. Guo, K. Zhou, X. Zhan and W. Ma, Adv. Mater., 2018, 30, 1805041 CrossRef.
- Y. Lin, Y. Jin, S. Dong, W. Zheng, J. Yang, A. Liu, F. Liu, Y. Jiang, T. P. Russell, F. Zhang, F. Huang and L. Hou, Adv. Energy Mater., 2018, 8, 1701942 CrossRef.
- Jeff L. Hernandez, N. Deb, R. M. W. Wolfe, C. K. Lo, S. Engmann, L. J. Richter and J. R. Reynolds, J. Mater. Chem. A, 2017, 5, 20687 RSC.
- Z. Li, L. Ying, P. Zhu, W. Zhong, N. Li, F. Liu, F. Huang and Y. Cao, Energy Environ. Sci., 2019, 12, 157 RSC.
- Z. Li, W. Zhong, L. Ying, N. Li, F. Liu, F. Huang and Y. Cao, Chin. J. Polym. Sci., 2019 DOI:10.1007/s10118-020-2356-3.
- X. Xu, T. Yu, Z. Bi, W. Ma, Y. Li and Q. Peng, Adv. Mater., 2018, 30, 1703973 CrossRef PubMed.
- B. Fan, W. Zhong, L. Ying, D. Zhang, M. Li, Y. Lin, R. Xia, F. Liu, H. L. Yip, N. Li, Y. Ma, C. J. Brabec, F. Huang and Y. Cao, Nat. Commun., 2019, 10, 4100 CrossRef PubMed.
- X. Gu, H. Yan, T. Kurosawa, B. C. Schroeder, K. L. Gu, Y. Zhou, J. W. F. To, S. D. Oosterhout, V. Savikhin, F. Molina-Lopez, C. J. Tassone, S. C. B. Mannsfeld, C. Wang, M. F. Toney and Z. Bao, Adv. Energy Mater., 2016, 6, 1601225 CrossRef.
- L. Zhang, B. Lin, B. Hu, X. Xu and W. Ma, Adv. Mater., 2018, 30, 1800343 CrossRef PubMed.
- K. Zhang, Z. Chen, A. Armin, S. Dong, R. Xia, H.-L. Yip, S. Shoaee, F. Huang and Y. Cao, Sol. RRL, 2018, 2, 1700169 CrossRef.
- B. Fan, X. Du, F. Liu, W. Zhong, L. Ying, R. Xie, X. Tang, K. An, J. Xin, N. Li, W. Ma, C. J. Brabec, F. Huang and Y. Cao, Nat. Energy, 2018, 3, 1051 CrossRef CAS.
- B. Fan, Z. Zeng, W. Zhong, L. Ying, D. Zhang, M. Li, F. Peng, N. Li, F. Huang and Y. Cao, ACS Energy Lett., 2019, 4, 2466 CrossRef CAS.
- S. Zhang, Y. Qin, J. Zhu and J. Hou, Adv. Mater., 2018, 30, 1800868 CrossRef.
- M. Zhang, W. Gao, F. Zhang, Y. Mi, W. Wang, Q. An, J. Wang, X. Ma, J. Miao, Z. Hu, X. Liu, J. Zhang and C. Yang, Energy Environ. Sci., 2018, 11, 841 RSC.
- J. Vogelsang, J. Brazard, T. Adachi, J. C. Bolinger and P. F. Barbara, Angew. Chem., Int. Ed., 2011, 50, 2257 CrossRef CAS.
- L. J. A. Koster, V. D. Mihailetchi, R. Ramaker and P. W. M. Blom, Appl. Phys. Lett., 2005, 86, 123509 CrossRef.
- J. Rivnay, S. C. Mannsfeld, C. E. Miller, A. Salleo and M. F. Toney, Chem. Rev., 2012, 112, 5488 CrossRef CAS PubMed.
- H. Lu, J. Zhang, J. Chen, Q. Liu, X. Gong, S. Feng, X. Xu, W. Ma and Z. Bo, Adv. Mater., 2016, 28, 9559 CrossRef CAS PubMed.
- N. Li, J. D. Perea, T. Kassar, M. Richter, T. Heumueller, G. J. Matt, Y. Hou, N. S. Guldal, H. Chen, S. Chen, S. Langner, M. Berlinghof, T. Unruh and C. J. Brabec, Nat. Commun., 2017, 8, 14541 CrossRef CAS PubMed.
- L. Zhu, W. Zhong, C. Qiu, B. Lyu, Z. Zhou, M. Zhang, J. Song, J. Xu, J. Wang, J. Ali, W. Feng, Z. Shi, X. Gu, L. Ying, Y. Zhang and F. Liu, Adv. Mater., 2019, 31, 1902899 CrossRef CAS PubMed.
- W. Zhao, S. Zhang, Y. Zhang, S. Li, X. Liu, C. He, Z. Zheng and J. Hou, Adv. Mater., 2018, 30, 1704837 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tc05358a |
| This journal is © The Royal Society of Chemistry 2020 |