Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Taking advantage of the disadvantage: employing the high aqueous instability of amorphous calcium carbonate to realize burst drug release within cancer cells
Cheng
Wang
,
Shaoqing
Chen
,
Qin
Yu
,
Fuqiang
Hu
and
Hong
Yuan
*
College of Pharmaceutical Sciences, Zhejiang University, 866 Yuhangtang Road, Hangzhou, 310058, China. E-mail: yuanhong70@zju.edu.cn
First published on 29th July 2020
Abstract
Correction for ‘Taking advantage of the disadvantage: employing the high aqueous instability of amorphous calcium carbonate to realize burst drug release within cancer cells’ by Cheng Wang et al., J. Mater. Chem. B, 2017, 5, 2068–2073, DOI: 10.1039/C6TB02826H.
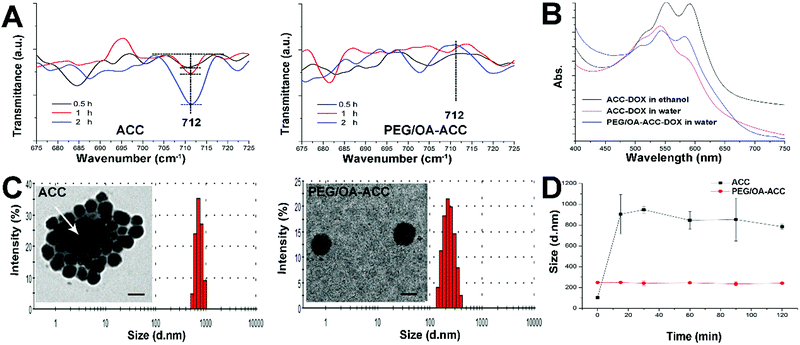
The authors regret that Fig. 2C (TEM image of PEG/OA-ACC) is incorrect due to mistakes in figure preparations. The correct version of Fig. 2 is shown below. The caption for this figure remains unchanged. It is noted that this was an honest mistake and does not affect the validity of the work and the conclusions. The authors apologise for any inconvenience caused.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2020 |