Target-directed enzyme-free dual-amplification DNA circuit for rapid signal amplification†
Abstract
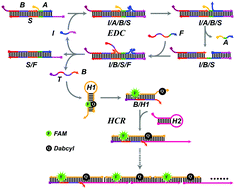
Dynamic DNA circuits have shown promising potential for amplified biosensing and bioengineering applications at the molecular level. Here, an enzyme-free, single-step and rapid signal amplification DNA circuit was developed by integrating target-directed entropy-driven catalysis (EDC) and hybridization chain reaction (HCR) for analysis of nucleic acids and small molecules. The target catalyzes the self-assembly of the EDC premade substrate complex and fuel strands to release the hidden amplicon trigger (T), which was encoded with trigger sequences for the downstream HCR circuit. The released T could motivate the successive cross-opening of HCR hairpins yielding long DNA nanowires and generated tremendously amplified fluorescence signals. Notably, this EDC–HCR circuit was driven by entropy without the requirement of any enzymes, thus greatly reducing the cost. The design of the hidden amplicon trigger (T) avoided the production of waste by-products and improved the reaction rate. Furthermore, as a modular circuit, we also demonstrated that our EDC–HCR cascade sensing system could be used as a versatile sensing platform for the highly sensitive and selective detection of other analysts, e.g. ATP in serum samples, through simply programming the reorganization sequences of the initiator. Therefore, the flexible and versatile EDC–HCR platform holds great potential in the fields of clinical diagnosis and biochemical analysis.
- This article is part of the themed collection: Journal of Materials Chemistry B HOT Papers


 Please wait while we load your content...
Please wait while we load your content...