Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Growth of large-scale MoS2 nanosheets on double layered ZnCo2O4 for real-time in situ H2S monitoring in live cells†
Veerappan
Mani
 *ab,
Shanthi
Selvaraj
cd,
Nithiya
Jeromiyas
*ab,
Shanthi
Selvaraj
cd,
Nithiya
Jeromiyas
 a,
Sheng-Tung
Huang
a,
Hiroya
Ikeda
d,
Yasuhiro
Hayakawa
a,
Sheng-Tung
Huang
a,
Hiroya
Ikeda
d,
Yasuhiro
Hayakawa
 d,
Suru
Ponnusamy
c,
Chellamuthu
Muthamizhchelvan
*c and
Khaled Nabil
Salama
d,
Suru
Ponnusamy
c,
Chellamuthu
Muthamizhchelvan
*c and
Khaled Nabil
Salama
 *b
*b
aInstitute of Biochemical and Biomedical Engineering, Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taipei 106, Taiwan, Republic of China
bSensors Lab, Advanced Membranes and Porous Materials Center, Computer, Electrical and Mathematical Science and Engineering Division, King Abdullah University of Science and Technology (KAUST), Saudi Arabia. E-mail: veerappan.mani@kaust.edu.sa; khaled.salama@kaust.edu.sa
cCentre for Nanoscience and Nanotechnology, Department of Physics and Nanotechnology, SRM Institute of Science and Technology, Tamil Nadu, India. E-mail: selvancm@gmail.com
dResearch Institute of Electronics, Shizuoka University, 3-5-1 Johoku, Naka-ku, Hamamatsu, Japan
First published on 1st July 2020
Abstract
There is an urgent need to develop in situ sensors that monitor the continued release of H2S from biological systems to understand H2S-related pathology and pharmacology. For this purpose, we have developed a molybdenum disulfide supported double-layered zinc cobaltite modified carbon cloth electrode (MoS2–ZnCo2O4–ZnCo2O4) based electrocatalytic sensor. The results of our study suggest that the MoS2–ZnCo2O4–ZnCo2O4 electrode has excellent electrocatalytic ability to oxidize H2S at physiological pH, in a minimized overpotential (+0.20 vs. Ag/AgCl) with an amplified current signal. MoS2 grown on double-layered ZnCo2O4 showed relatively better surface properties and electrochemical properties than MoS2 grown on single-layered ZnCo2O4. The sensor delivered excellent analytical parameters, such as low detection limit (5 nM), wide linear range (10 nM–1000 μM), appreciable stability (94.3%) and high selectivity (2.5-fold). The practicality of the method was tested in several major biological fluids. The electrode monitors the dynamics of bacterial H2S in real-time for up to 5 h with good cell viability. Our research shows that MoS2–ZnCo2O4–ZnCo2O4/carbon cloth is a robust and sensitive electrode to understand how bacteria seek to adjust their defense strategies under exogenously induced stress conditions.
1. Introduction
Most of the current sensors are normal sensors that can provide static information about a biological parameter at a certain time instant. However, many of the biological parameters are actually dynamic variables; their amount varies over time and depends on external stimuli. These biological variables require real-time sensors that can give continuous information at different times.1–3 Hydrogen sulfide (H2S) is one such dynamic parameter that plays significant roles in biological cells in regulating physiologically essential processes as a second chemical messenger.4 H2S is essential to modulate neurotransmission and relax muscles5 and it exerts cardioprotective, anti-inflammatory, and anti-apoptotic effects on several organs including cardiovascular, central nervous system (CNS), and gastrointestinal systems.6 The normal levels of endogenous H2S productions are 10–100 μM and 50–160 μM in blood and CNS, respectively.7 H2S injections in controlled doses actually have potential therapeutic effects, so slow releasing H2S drugs have the potential to treat certain cardiovascular, neurological, and carcinogenic diseases. However, abnormal H2S levels are extremely detrimental and implicated in many diseases, such as Alzheimer's disease, chronic kidney disease, liver cirrhosis, and traumatic brain injury.8,9 Serum H2S level is a biomarker for cardiovascular diseases such as atherosclerosis, coronary heart disease, hypertension and chronic obstructive pulmonary disease.10 Therefore, a sensitive real-time H2S tracking tool is essential to continuously measure its release from biological media.11 Electroanalytical methods can provide quantitative information about analytes in biochemical media with high spatial and temporal resolution for hours or even days, and are simple, cost-effective and derivatization-free.12–14 Nevertheless, low sensitivity, selectivity, and inadequate reproducibility of the unmodified electrode resulting from poor surface properties of the bulk surface limit the full potential of electroanalytical methods.15 The design and synthesis of advanced nanostructured materials is a facile method to tailor the surface properties of the electrodes.16 Often, integrated hybrid electrode materials can meet the requirements of real-time in situ electrocatalytic sensors by combining the properties of all its components.17,18Zinc cobaltite (ZnCo2O4), a mixed ternary metal oxide nanostructure, has attracted considerable interest in electrochemical applications due to its multiple redox reactions, expanded surface area, stability, and increased conductivity.19,20 ZnCo2O4 has a similar cubic spinal crystal structure to Co3O4, and exhibits relatively better electrocatalytic activity, lower cost, and environmental benignity than Co3O4.21 ZnCo2O4 can be prepared in different shapes such as, nanotubes,22 microspheres,23 nanoflowers,24 and core–shell structures.25 Besides, the hybridization of ZnCo2O4 with metal sulfides can produce efficient functional engineering materials with synergistic properties.26,27 Although the applicability of such ZnCo2O4 materials has been demonstrated in energy devices, their use in biosensors is rarely explored.28,29 On the other hand, electrode passivation is a major issue in fabricating reproducible H2S sensors.30 A surface preconditioning procedure is reported to eliminate the passivation issue by placing a layer of elemental sulfur on the electrode prior to measurement.15 Here, we present an alternative approach based on the inherent property of molybdenum sulfide (MoS2) material. We suggest that the presence of sulfur layers in MoS2 sheets can help to overcome the sulfur deposition through repulsive interactions.8 Unlike previous methods, this approach does not affect the conductivity of the working electrode. Moreover, MoS2 nanosheets and its composites are critically acclaimed materials for electrocatalytic sensing applications.31
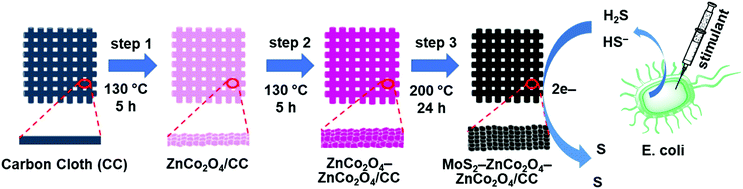
Here we report a synthesis flow for hierarchical MoS2 decorated double-layered ZnCo2O4 nanostructures on top of a carbon cloth electrode for robust and sensitive H2S detections. Carbon cloth is an inexpensive and flexible template with high mechanical strength. Its interconnected network assembly can provide rich active spots to grow nanomaterials.32 As shown in Fig. 1, single-layered and double-layered ZnCo2O4 nanostructures were fabricated on a CC substrate. Then, a uniform layer of MoS2 nanostructure was deposited to obtain MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC electrodes. Our studies show that MoS2–ZnCo2O4–ZnCo2O4/CC has relatively better surface properties and superior electrocatalytic ability compared to other electrodes. In addition, it displays several advantages over other materials, such as minimized overpotential, low detection limit, no pre-treatment, and functioning at physiological pH. MoS2–ZnCo2O4–ZnCo2O4/CC can be used to establish a sensing platform for tracking H2S release in E. coli. As the electrode is based on flexible carbon fabric, it can be a suitable candidate for wearable biosensing applications.33
2. Experimental
2.1. Materials and instrumentation
All the purchased chemicals were of analytical grade and used directly without any further purification. Zinc nitrate hexahydrate [Zn(NO3)2·6H2O], cobalt nitrate hexahydrate [Co(NO3)2·6H2O], urea [CO(NH2)2], ammonium fluoride [(NH4)F], sodium molybdate [Na2MoO4·6H2O], and thiourea [CH4N2S] were purchased from Wako chemicals, Japan. Carbon cloth was provided by sainergy fuel cell India. The aqueous solution of H2S was freshly prepared on the day of use by dissolving NaHS (purchased from Sigma) in deoxygenated PBS, sealing the container with a rubber septum, and purging the headspace with N2. Instead of NaHS, the term H2S has been used throughout the manuscript for clarity. A human blood sample was collected from a healthy human with consent. 0.1 M phosphate buffer saline (PBS), pH 7.4 was used as a supporting electrolyte. 0.1 M acetate buffer (with pH 4.4 & 5.4), 0.1 M phosphate buffer (with pH 6.4), 0.1 M PBS (with pH 7.4), and 0.1 M Tris-buffer saline (with pH 8.4 & 9.4) were used for a pH optimization test. All studies were performed three times and the average of three readings was used to plot the data.The electrochemical experiments were performed with a CHI 612D electrochemical workstation (CH Instruments, Inc., U.S.A). FE-SEM characterization were performed using a JSM-7001F instrument. X-ray diffraction (XRD) studies were performed using an RINT-2200 diffractometer, Rigaku, Japan, CuKα radiation, λ = 1.54178 Å. Raman spectra were acquired using NRS-7100 with a laser excitation wavelength of 532 nm (spot size 1 μm). An EIM6ex Zahner (Kronach, Germany) was used for electrochemical impedance spectroscopy (EIS) studies. EIS parameters are, bias potential = 0 V, amplitude = 5 mV, and frequency = 100 mHz to 100 kHz. X-ray photoelectron spectra (XPS) were obtained by using a Shimadzu ESCA 3100. The counting of bacterial cells was performed using a spectrophotometer fitted with a xenon lamp and 1.0 cm quartz cells.
2.2. Fabrication of single layered ZnCo2O4 nanosheets on carbon cloth – step 1
Carbon cloth (CC: 2 cm × 2 cm) was pre-cleaned with 3 M HCl solution for about 10 min to remove exterior filth and cleaned with DI water & ethanol for about thrice the time. In the fabrication process, 3 mM Zn(NO3)2·6H2O, 6 mM Co(NO3)2·6H2O, 10 mM NH4F and 15 mM Co(NH2)2 were liquified in 80 mL DI water and stirred for about 30 min to attain the homogeneous mixture solution. The prepared homogeneous mixture solution was moved to a Teflon-lined stainless steel autoclave with the pre-cleaned CC substrate and sustained at 130 °C for 5 h. After cooling down to room temperature, the fabricated ZnCo2O4/CC was washed with DI water and ethanol and dried at 90 °C overnight. After the drying process, the electrode was subjected to a calcination process at 200 °C for 5 h under atmospheric conditions.2.3. Fabrication of double layered ZnCo2O4 nanosheets on carbon cloth – step 2
In the fabrication process, 3 mM Zn(NO3)2·6H2O, 6 mM Co(NO3)2·6H2O, 10 mM NH4F and 15 mM Co(NH2)2 were liquified in 80 mL DI water and stirred for about 30 min to attain the homogeneous mixture solution. The prepared homogeneous solution was transferred to a Teflon lined stainless steel autoclave with the ZnCo2O4/CC and maintained at 130 °C for 5 h. After cooling down to room temperature, the fabricated ZnCo2O4–ZnCo2O4/CC was prudently washed with DI water & ethanol several times and dried at 90 °C overnight. After drying, the electrode was subjected to calcination at 200 °C for 5 h.2.4. Fabrication of MoS2 nanosheets–ZnCo2O4–ZnCo2O4 on carbon cloth – step 3
In the fabrication process, 2 mM Na2MoO4·6H2O and 13 mM CH4N2S were liquefied in 80 mL DI water and stirred for about 30 min to attain a homogeneous mixture. The mixture was transferred to a Teflon-lined stainless steel autoclave with fabricated electrode material ZnCo2O4–ZnCo2O4/CC and sustained at 200 °C for about 24 h. After cooling down to room temperature, the fabricated MoS2–ZnCo2O4–ZnCo2O4/CC was washed with water and ethanol several times and dried at 90 °C overnight. After the drying process, the fabricated electrode materials were subjected to calcination at 200 °C for 5 h to yield MoS2–ZnCo2O4–ZnCo2O4/CC. In parallel, MoS2 was also deposited on a single-layered ZnCo2O4 to prepare MoS2–ZnCo2O4/CC.Finally, a thin layer of polymerized o-phenylenediamine (POPD) was deposited on the MoS2–ZnCo2O4–ZnCo2O4/CC through voltammetric deposition. This coating is useful to enable anti-fouling properties against biological species and to provide H2S permselective properties to the electrode.15 To perform this step, the electrode (MoS2–ZnCo2O4–ZnCo2O4/CC or MoS2–ZnCo2O4/CC) was transferred into an electrochemical cell containing 10 mM o-phenylenediamine suspended in PBS (pH 7.4) and 2 cycles of cyclic voltammograms were ramped at 0.025 V s−1 scan rate between 0 V and 1.0 V. The POPD coated electrode was carefully washed with water and dried under ambient conditions before being used for sensing study.
2.5. Bacteria cell culture
E. coli strain MG1655 was grown on a Lysogeny broth (LB) agar plate. The growth period was overnight and the temperature was maintained at 37 °C. A single colony of the as-grown E. coli was inoculated in 20 mL of LB medium and the incubation was maintained overnight at 37 °C. Constant shaking was applied for both the incubation processes. The cell counts were estimated by spectrophotometer analysis (optical density, OD600 = 0.65 at 37 °C). The bacterial solution was diluted with fresh LB medium with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. The bacterial count was calculated to be 2.5 × 109 cells per mL via cytometry.
9. The bacterial count was calculated to be 2.5 × 109 cells per mL via cytometry.
2.6. Electrochemical experiments in E. coli
2.5 × 109 cells per mL of E. coli in a 20 mL LB medium (pH 7.4) was transferred to a typical electrochemical cell. Three electrodes were immersed in the cell: MoS2–ZnCo2O4–ZnCo2O4/CC was the working electrode, Ag/AgCl was the reference electrode and Pt was the counter electrode. The chronoamperometric experiments were conducted at a constant potential of +0.20 V for 100 s. The steady-state currents were used to make calibration plots.3. Results and discussions
The schematic representation for the fabrication process of hierarchical nanoarchitectures of MoS2–ZnCo2O4–ZnCo2O4 on flexible carbon cloth is illustrated in Fig. 1. The mechanism of nucleation and the subsequent growth process of the ZnCo2O4 nanosheets are represented below. Initially, the ZnxCo2x(OH)6x precursor nanoparticles were produced by the hydrolysis of urea (Co(NH2)2), during the hydrothermal process of dissociation of Zn2+ and Co2+ ions with OH− ions. The formed ZnxCo2x(OH)6x precursor nanoparticles may stick to the flexible CC substrate and be loaded upon one another. Then, the formed ZnxCo2x(OH)6x precursor nanoparticles grew into thin layered nanosheets, which is driven by the minimization of the surface energy. The ZnxCo2x(OH)6x precursor nanoparticles can be easily transformed to ZnCo2O4 through the subsequent annealing process. The as-deposited thin single and double layered ZnCo2O4 nanosheets were used as a template, by providing plentiful active nucleation spots for successive progress of MoS2.By the ensuing hydrothermal process with MoS2 precursor solution from Na2MoO4·6H2O and CH4N2S, MoS2 nanocrystals were initially formed on the surface of thin single and double layered ZnCo2O4 nanosheets. Later, these MoS2 nanocrystals further grew into interconnected MoS2 nanosheets, resulting in the formation of nanoarchitectures on a flexible CC substrate. The possible mechanism for the formation of MoS2–ZnCo2O4– ZnCo2O4/CC is shown in the following equations:34
The formation of ZnCo2O4 is represented as:
| Co(NH2)2 + H2O → C3H6N6 + 6NH3 + 3CO2 | (1) |
| NH3 + NH4F + H2O → 2NH4+ + OH− + F− | (2) |
| xZn2+ + 2xCo2+ + 6xOH− → ZnxCo2x(OH)6x | (3) |
| ZnxCo2x(OH)6x + 0.5xO2 → xZnCo2O4 + 3xH2O | (4) |
| SC(NH2)2 + 2H2O → CO2 + 2NH3 + H2S | (5) |
| H2S → 2H+ + S2− | (6) |
| 4MoO42− + 9S2− + 24H+ → 4MoS2 + 12H2O + SO42− | (7) |
3.1. Improved surface properties of MoS2–ZnCo2O4–ZnCo2O4/CC over MoS2–ZnCo2O4/CC
Fig. 2A and B display the FE-SEM image of single-layered ZnCo2O4 nanosheets on carbon cloth, which clearly shows that the entire CC is fully covered with uniform and aligned thin ZnCo2O4 nanosheets. Fig. 2C and D show the FE-SEM image of ZnCo2O4–ZnCo2O4 hierarchical nanosheets. The aligned double-layered ZnCo2O4 nanosheets are much denser, uniform and homogeneous than the aligned single-layered ZnCo2O4 nanosheets. In addition, the surface of the double-layered ZnCo2O4 nanosheets is rougher than the single-layered ZnCo2O4 nanosheets. The inner layer of ZnCo2O4 and outer layer ZnCo2O4 are expected to have different dimensions and aspect ratios, because of the utilization of different substrates as templates; i.e., CC and ZnCo2O4/CC, during the step-1 and step-2 hydrothermal approaches. No ZnCo2O4 was crammed in the inter-space region of the nanostructure-interconnected assembly, signifying that outer ZnCo2O4 layer was fully-fledged on the exterior of the innermost ZnCo2O4 thin nanosheets.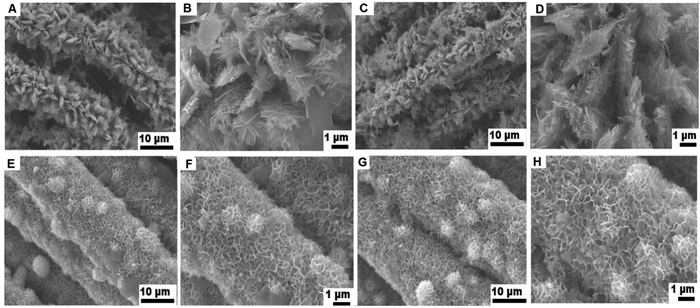 | ||
| Fig. 2 FE-SEM images of single-layered ZnCo2O4/CC (A and B), double-layered ZnCo2O4–ZnCo2O4/CC (C and D), MoS2–ZnCo2O4/CC (E and F) and MoS2–ZnCo2O4–ZnCo2O4/CC (G and H). | ||
Fig. 2E and F show the FE-SEM images of MoS2-single layered ZnCo2O4 nanosheets attained through the step-3 hydrothermal approach, with the aligned thin single layered nanosheets like ZnCo2O4/CC as the substrate. Fig. 2G and H display the typical FE-SEM image of MoS2–ZnCo2O4–ZnCo2O4 nanoarchitectures accomplished through the step-3 hydrothermal approach, with the aligned thin double-layered ZnCo2O4–ZnCo2O4/CC as the substrate. Large scale interconnected MoS2 nanosheets grew uniformly and densely on both the single layered and double layered ZnCo2O4 nanosheets on a CC substrate. With the help of ZnCo2O4 nanosheets, the interconnected MoS2 can assemble on both sides of the ZnCo2O4 sheets in hierarchical porous overlays. Such hybrid nanosystems can efficiently facilitate charge transport at the interface.
3.2. Crystal structure, elemental analysis, and binding studies
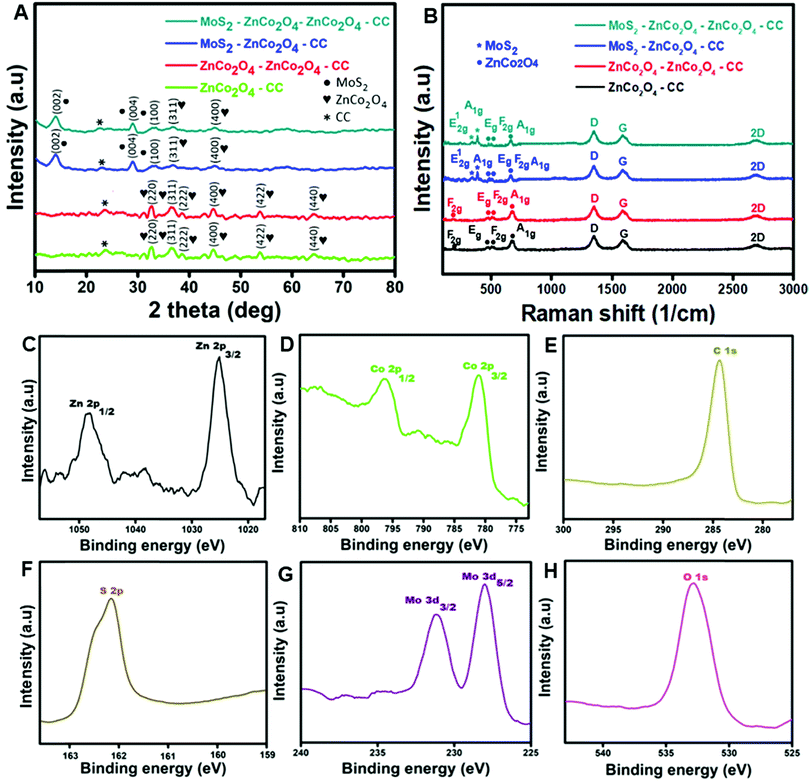
Fig. 3A displays the XRD patterns of ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC. From the XRD peak patterns of ZnCo2O4/CC and ZnCo2O4–ZnCo2O4/CC, the diffraction crystal planes (311), (400), (220), (222), (422), and (440) could be indexed to a cubic crystal spinel structure of ZnCo2O4 (JCPDS No. 23-1390).35 From the XRD peak patterns of MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC, the diffraction crystal planes (002), (004) and (100) could be indexed to MoS2 nanostructures (JCPDS No. 37-1492). No other peaks indexed to impurities are detected from the XRD curves, indicating the formation of an electrode with high crystallization and purity.Fig. 3B displays the Raman spectra of ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC. Four peaks at 195, 474, 510 and 679 cm−1 were observed in the Raman spectra of ZnCo2O4/CC and ZnCo2O4–ZnCo2O4/CC, assigned to the characteristic modes of ZnCo2O4 nanostructures with the vibrational modes of F2g, Eg, F2g and A1g, respectively.26 An additional two peaks are observed for the Raman spectra of MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC at 382.4 and 407.6 cm−1, which are recognized as the E12g and A1g vibration modes of MoS2, respectively.36
Fig. 3C–H depict the XPS analysis of the MoS2–ZnCo2O4–ZnCo2O4/CC electrode material. From Fig. 3C, the electronic configuration of Zn 2p identified at 1025.3 (Zn 2p3/2) and 1048.3 (Zn 2p1/2) eV, with an energy level difference of about 23.0 eV substantiated the presence of Zn ions with 2+ oxidation states.37 From Fig. 3D, the electronic configuration of Co 2p acknowledged at 781 and 797 eV is assigned to Co 2p3/2 and Co 2p1/2, respectively. From Fig. 3E, the standard carbon peak achieved at 284.7 eV can be allocated to C 1s spectra. From Fig. 3F, a strong peak appeared at 162.2 eV assigned to S 2p spectra. From Fig. 3G, the peaks attained at 33 and 229 eV, are assigned to Mo 3d3/2 and Mo 3d5/2.38 From Fig. 3H, a strong peak obtained at 532 eV is allocated to O 1s spectra.
3.3. Voltammetric behavior of the electrodes
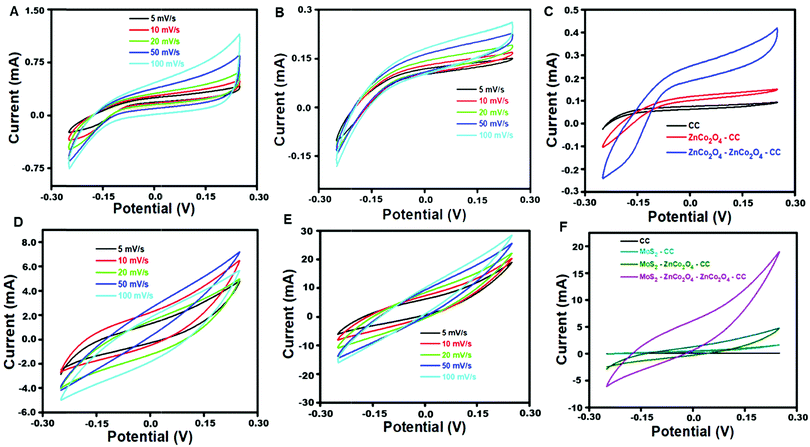
Fig. 4A and B display the CV curves of ZnCo2O4–CC and ZnCo2O4–ZnCo2O4/CC, at an applied potential window between −0.25 V and +0.25 V with varying scan rates from 5 to 100 mV s−1. 0.1 M KOH was used as a supporting electrolyte. All the CV curves displayed a pseudocapacitive nature and the redox peaks are mostly generated owing to the faradaic electrochemical reactions that are related to the representation as M-O/M-O-OH, where M is denoted as Zn2+ and Co2+, which are associated with the OH− anions.39 The eqn (8)–(10) express the possible redox reactions that occur during the electrochemical process at the outer surface of MoS2–ZnCo2O4–ZnCo2O4/CC.| ZnCo2O4 + H2O + e− ↔ 2CoOOH + Zn(OH)− | (8) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (9) |
| ZnOH + OH− ↔ Zn(OH)2 + e− | (10) |
The CV area for the double-layered ZnCo2O4 electrode was much larger than that of the single-layered ZnCo2O4, revealing that the double-layered ZnCo2O4/CC hierarchical electrode has higher electrochemical activity than the single layered ZnCo2O4/CC (Fig. 4C).40 A thin outer layer of porous ZnCo2O4 with enormous surface area favors fine ion diffusion at the electrolyte/electrode interface, which can provide more electrochemical active surface area for electrochemical reactions. Because of the rational design of double-layered ZnCo2O4/CC, it could achieve the greatest use of each layer of active material. Besides, the CV areas for the fabricated electrode materials are entirely different, because of the polarization effect, which is thoroughly linked to the structural morphology of the electrode materials.41 Additionally, the resulting synergy effect of the double layered ZnCo2O4/CC hierarchical electrode affords an efficient pathway for ion and electron transport, during the electrochemical analysis.
Fig. 4D and E display the CV curves of MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC with varying scan rate measurement values from 5 to 100 mV s−1. 0.1 M KOH was used as a supporting electrolyte. CV curves display quasi-rectangular shapes, demonstrating the presence of dual electrochemical behavior of both electrochemical double layer capacitance and the pseudocapacitive nature of the fabricated electrode materials. No redox peaks were observed in the CV curves obtained with a KOH electrolyte, because of the development of a double layer at the interface of the electrode and electrolyte. Additionally, the CV area for electrode MoS2–ZnCo2O4–ZnCo2O4/CC was much larger than that of the MoS2–ZnCo2O4/CC, revealing that the double layered ZnCo2O4/CC has higher electrochemical activity than the single-layered ZnCo2O4/CC, as expressed in Fig. 4F. During the electrochemical process, the K+ ions may diffuse into the surface of a MoS2 layer as represented in eqn (11),
| (MoS2) surface + K+ + e− ↔ (MoS2 + K+) surface + e− | (11) |
Moreover, a CV rectangular curve with slight variation in the fabricated hybrid electrodes, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC for all scan rate measurements is primarily owing to reversible redox reactions of Mo3+/Mo2+ linked with the OH− ions. Eqn (12) and (13) show the possible redox reactions of MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC,
| MoS2 + OH− ↔ MoSOH + e− | (12) |
| MoSOH + OH− ↔ MoSO + H2O + e− | (13) |
The electrochemically effective surface areas of ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC have been calculated by studying their electrochemical properties in the presence of K3[Fe(CN)6] redox mediator. The Randles–Sevcik equation was used to calculate the active surface areas.42,43 The active surface areas of ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC were found to be 0.672 cm2, 0.750 cm2, 0.842 cm2, and 0.984 cm2 respectively. MoS2–ZnCo2O4–ZnCo2O4/CC has the largest active surface in comparison with other electrodes, which is in line with CV and EIS results.
3.4. MoS2–ZnCo2O4–ZnCo2O4/CC has excellent interfacial electron transfer properties
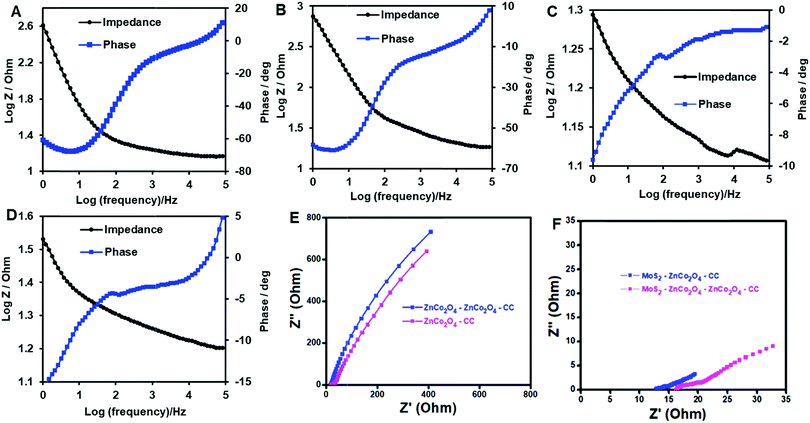
Fig. 5 displays the EIS-Bode plot analysis of ZnCo2O4/CC (A), ZnCo2O4–ZnCo2O4/CC (B), MoS2–ZnCo2O4/CC (C) and MoS2–ZnCo2O4–ZnCo2O4/CC (D). From Fig. 5A–D, at 1 Hz frequency, the attained phase angles of ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC are represented as 60.9°, 58.5°, 19.68° and 15.6°, respectively. During the electrochemical process, larger phase shift values are observed for the electrodes, which is due to the charge transfer mechanism of different kinds of active materials.44 The MoS2–ZnCo2O4–ZnCo2O4/CC electrode showed better phase angle compared to other electrodes, suggesting superior electron transfer at this interface. Fig. 5E and F compare the Nyquist plots of ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC. The attained equivalent series resistance (ESR) values for the electrode materials ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC are, 2.9 Ω, 2.4 Ω, 1.3 Ω and 0.24 Ω, respectively. These electrodes have excellent electronic conductivity, as the ZnCo2O4 and MoS2 arrays tightly attached to the carbon cloth to form very good adhesion and electrical contact, creating a smoother pathway for interface electron transfer. While comparing the ESR values of the above fabricated electrode materials, the MoS2–ZnCo2O4–ZnCo2O4 has exhibited lower resistance values than other electrode materials, revealing the fast electron transfer at this interface. Thus, the double-layered ZnCo2O4 facilitates better electrochemical properties for the electrode compared to single layered ZnCo2O4, which is also in agreement with surface morphology results.3.5. MoS2–ZnCo2O4–ZnCo2O4/CC: an excellent electrocatalyst for H2S oxidation
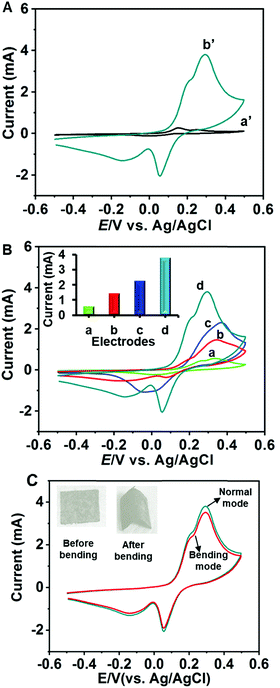
Next, the H2S sensing aptitudes of the electrodes were tested by cyclic voltammetry. The potential was cycled between −0.50 V and + 0.50 V with a scan rate of 50 mV s−1. Fig. 6A shows the CVs of MoS2–ZnCo2O4–ZnCo2O4/CC in the absence (curve a′) and presence (curve b′) of 1 mM H2S. In the absence of H2S, the CV of MoS2–ZnCo2O4–ZnCo2O4/CC displayed peaks corresponding to characteristic redox reactions of the nanomaterial, as explained in eqn (8)–(10). However, a large increase in peak current at +0.30 V with an onset potential of + 0.20 V was observed in the presence of H2S. The dissolved H2S and HS− are the major forms of H2S at pH 7.4 and fortunately both of them are electrochemically active.15 They can undergo a two-electron oxidation process as given in eqn (14) and (15).| H2S → S0 + 2e− + 2H+ | (14) |
| HS− → S0 + 2e− + H+ | (15) |
Fig. 6B compares the electrocatalytic responses of MoS2–ZnCo2O4–ZnCo2O4/CC with unmodified CC, ZnCo2O4–ZnCo2O4/CC, and MoS2–ZnCo2O4/CC. The bar diagram presented as an inset to Fig. 6B compares the response currents of the electrodes. The H2S oxidation peak current at MoS2–ZnCo2O4–ZnCo2O4/CC was 0.5, 1.4, and 2.2 folds higher than the MoS2–ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC and unmodified CC, respectively. The oxidation potential at MoS2–ZnCo2O4–ZnCo2O4/CC was 0.33 V, 0.35 V, and 0.37 V lower than that at MoS2–ZnCo2O4/CC, ZnCo2O4–ZnCo2O4/CC and unmodified CC, respectively. The minimized overpotential is highly useful in constructing a sensor without interference from common biological species. Notably, MoS2–ZnCo2O4–ZnCo2O4/CC showed relatively better performance than the MoS2–ZnCo2O4/CC, which indicates the significant role of double-layered ZnCo2O4. In accordance with the SEM and voltammetric results, we infer that the improved surface properties, such as surface area, roughness and porosity are the major reasons for the improved electrochemical properties. A typical electrochemical-coupled chemical (EC) reaction mechanism is proposed in the literature for H2S oxidation at chemically modified electrodes such as MoS2–ZnCo2O4–ZnCo2O4/CC.45 The possible electrocatalytic mechanism involving the active nanomaterials can be given as eqn (16)–(19),
At MoS2 sites,
| Mo(IV)S2 + H2S ↔ Mo(II)S2 + S | (16) |
At ZnCo2O4 sites,
| ZnCo2O4 + H2O + e− ↔ CoOOH + Zn(OH)− | (17) |
| CoOOH + H2S ↔ CoO2 + S + OH− | (18) |
| ZnOH + OH− ↔ Zn(OH)2 | (19) |
Next, the ability of the electrode to determine H2S under bending conditions was tested. Fig. 6C shows the CV curves of MoS2–ZnCo2O4–ZnCo2O4/CC towards 1 mM H2S under normal and bending conditions. The electrode was bent by twisting to 180 degrees 20 times to make bending deformation. About 95% of the initial H2S oxidation peak current was retained for the bending electrode compared to the normal electrode, which suggests that the electrode has the ability to produce reproducible signals under strained conditions. These results indicate good flexibility and mechanical stability of the electrode, which can be correlated with the properties of carbon cloth and mechanical adhesion of the material with a carbon cloth electrode. The electrode can be used to fabricate flexible H2S sensors.
A high stability electrode is needed for continuous monitoring because the electrode has to be in contact with the analyte for hours or even days. To investigate the stability of MoS2–ZnCo2O4–ZnCo2O4/CC, its H2S sensing capacity was monitored every day by analyzing catalytic responses periodically. The electrode showed approximately 94.3% of its initial current response after the tenth day of its continuous use, suggesting appreciable stability (Fig. S1A, ESI†). The stability result suggests that the material has good mechanical adhesion on the surface of carbon cloth. It is known that direct growth of materials on the carbon cloth electrode can provide good mechanical adhesion and excellent conductivities, which in turn enable high selectivity for the resulting sensor.46,47 Reproducibility of the MoS2–ZnCo2O4–ZnCo2O4/CC was tested by conducting CV experiments towards 1 mM H2S. The sensor displayed good reproducibility with an R.S.D. of 3.9% for five individual electrodes (Fig. S1B, ESI†). A possible explanation for the appreciable reproducibility and anti-fouling property of the electrode can be postulated in terms of repulsive forces between the sulfur layers of MoS2 and the elemental sulfur of the H2S-oxidized product. MoS2 sheets contain a layer of Mo atoms sandwiched between two layers of S atoms.48 When elemental sulfur produced by H2S oxidation reaches the electrode surface, it confronts with the sulfur layers of MoS2 at the interface region, where it is most likely to experience a repulsive interaction. This interaction imposes the elemental sulfur to divert to the bulk solution region, thus preventing the fouling of the interface region.
The effects of H2S concentration, scan rate, and pH are given in the ESI.† The effect of concentration confirms good anti-poisoning nature of the film (Fig. S2A and B, ESI†). The effect of scan rate suggests a diffusion controlled oxidation process (Fig. S3A and B, ESI†). The effect of pH suggests that pH 7.4 is the optimum pH for maximum H2S sensing performance (Fig. S4A–C, ESI†).
3.6. High sensitivity and selectivity
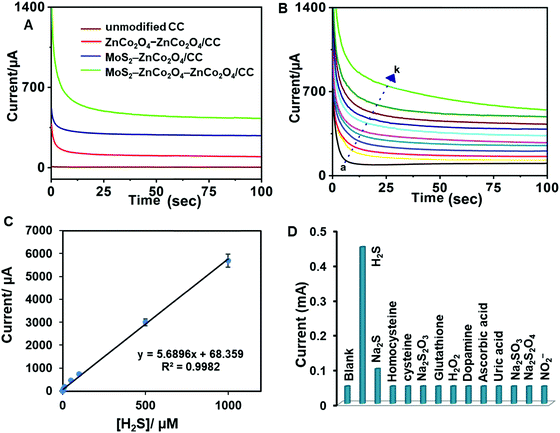
A chronoamperometric method was used to investigate the H2S determination capabilities of unmodified CC, ZnCo2O4–ZnCo2O4/CC, MoS2–ZnCo2O4/CC and MoS2–ZnCo2O4–ZnCo2O4/CC. The electrode potential was +0.20 V (vs. Ag/AgCl). As shown in Fig. 7A, the MoS2–ZnCo2O4–ZnCo2O4/CC displayed superior chronoamperometric response to sense H2S compared to the control electrodes. Next, the response of MoS2–ZnCo2O4–ZnCo2O4/CC toward different concentrations of H2S was tested (Fig. 7B). Well-defined and quick responses were obtained for each concentration of H2S and a good linear relationship was observed between current and [H2S]. Next, chronoamperometric experiments were conducted from the nanomolar to millimolar range to find the sensor's working range, which was found to be 10 nM–1000 μM (Fig. 7C). The limit of detection (LOD) was estimated to be 5 nM. The LOD was calculated using the formula LOD = 3sb/S (where sb = standard deviation of 10 blank signals and S = sensitivity).As can be seen from Table 1, the MoS2–ZnCo2O4–ZnCo2O4/CC shows relatively better performance than many of the existing methods. This is mainly because the double-layered ZnCo2O4 and MoS2 nanostructured materials provide large surface area, and excellent electrocatalytic and electron shuttling properties. Although the electrode potentials of enzymatic biosensors are slightly better than our method, their use in continuous monitoring systems is still limited because fragile bio-components negatively affect the sensor's life.53 Some reports use highly acidic or alkaline pH conditions; but these extreme treatments cause false positives or false negatives.56 Besides, most of the previous reports mainly focused on environmental or serum samples, but were not used in live biological systems such as E. coli. In contrast, the MoS2–ZnCo2O4–ZnCo2O4/CC sensor has several advantages, such as working at decent potential, operating at physiological pH, and detecting in situ H2S from live biological media.
| Electrode (amperometry technique) | Linear range/μM | Detection limit | Real samples | Ref. |
|---|---|---|---|---|
| Graphene-based 3D scaffold | 0.2–10 | 50 nM | HeLa cells | 49 |
| Curcumin-quinone/carbon black | 10–1200 | 7.12, 2.40 μM | Tap water | 50 |
| 10–100 | ||||
| Pencil graphite electrode/quercetin | 1–20, 20–800 | 0.3 μM | Waste water | 51 |
| 9,10-Phenanthrenequinone/graphene oxide | 1–100, 300–5000 | 700 nM | Human blood | 52 |
| Coprinus cinereus peroxidase/chitosan/SPE biosensor | 1.09–16.3 | 0.3 μM | Environmental water | 53 |
| Cu2O–CuO@Au | 0.01–11000 | 1 nM | Human cell A375 | 54 |
| V2O5/PtIr wire | 0.5–15 | 0.5 μM | — | 55 |
| MoS2–ZnCo2O4–ZnCo2O4/CC | 0.01–1000 | 5 nM | E. coli, human whole blood, fetal bovine serum | This work |
Next, the selectivity of the MoS2–ZnCo2O4–ZnCo2O4/CC was tested by monitoring its sensing performance in the presence of likely interfering agents that are commonly coexisting in biological media (Fig. S5, ESI†). 100 μM H2S and 250 μM of homocysteine, cysteine, Na2S2O3, glutathione, H2O2, dopamine, ascorbic acid, uric acid, Na2SO3, Na2S2O4, NO2−, and NO have been used. From the bar chart displayed in Fig. 7D, we infer that the electrode responded exclusively to H2S, but not to other compounds. Irrespective of their 2.5-fold excessive presence in the medium, the signal contribution of other analytes is less than 5%. The selective nature of the film can be explained based on the properties of the materials, which are elaborated below:
(1) The thin coating of POPD provides selective permeability to H2S. H2S can penetrate through the film due to its size matching with the pores of POPD. Thiols and other biological species are unable to permeate the POPD film because of their large sizes.57 It is worth mentioning that, traditional ion selective electrodes encounter severe interference by wrongly counted biothiols.56 Interestingly, the described electrode eliminates the signal from such biothiols.
(2) The film retains a net positive charge, which rejects positively charged molecules such as dopamine and uric acid via electrostatic repulsive interactions.
(3) H2S at unmodified electrodes usually requires high positive potentials (>+0.4 V) for oxidation, but higher potential allows interference from unwanted biological species. H2S oxidation at MoS2–ZnCo2O4–ZnCo2O4/CC occurs at a minimized overpotential, +0.20 V vs. Ag/AgCl. On the other hand, a previous report demonstrated that POPD has permeability to H2O2; in contrast, here we do not see any notable signal for H2O2.57 This is because of the differences in electrode potential requirements between H2O2 and H2S. Although H2O2 has the ability to reach the electrode, it requires high positive potentials. Thus, the use of a minimized overpotential has contributed significantly to the electrode's selectivity.
3.7. Practical applicability in whole blood, fetal bovine serum and E. coli
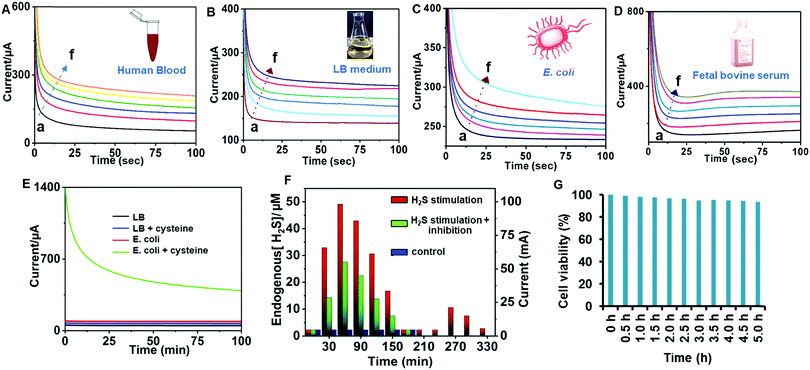
The practical sensing performance of MoS2–ZnCo2O4–ZnCo2O4/CC demonstrated in human blood (A), LB medium (B), E. coli (C) and fetal bovine serum (D) have been tested (Fig. 8). Whole samples were tested without any pre-treatments. These media have been found to be H2S-free and then known amounts of H2S (i.e., NaHS) were spiked and the resulting spiked samples were analyzed by chronoamperometry. Regardless of the presence of other biological compounds in the aforementioned biological fluids, our method can accurately measure the amount of H2S produced, indicating its best practical applicability in major biological fluids.3.8. Real-time tracking of H2S production in E. coli
H2S is endogenously produced by bacterial cells as a common defense agent against antibiotics.58 H2S protects the bacteria from oxidative stress and antibiotics by suppressing DNA breakdown and enhancing the activity of catalase and superoxide dismutase.59 It is well known that H2S is biosynthesized from cysteine; therefore cysteine can be used as a stimulant to induce the cells to produce H2S. Fig. 8E displays the chronoamperometric responses of the MoS2–ZnCo2O4–ZnCo2O4/CC to E. coli in the presence (green curve) and absence (red curve) of cysteine. A steady background current was observed in the absence of stimulant; however, a sharp increase in the chronoamperometric current was observed with stimulant. Here, the cysteine supplementation stimulated the E. coli to produce H2S endogenously, and the as-produced H2S transported to the interface via diffusion and subsequently electrocatalyzed by the interface. The amount of H2S release is directly proportional to the response current. Control experiments such as analysis in only LB medium and LB medium + cysteine have shown just baseline.Next, the MoS2–ZnCo2O4–ZnCo2O4/CC was employed to track dynamic endogenous H2S production with respect to stimulation time. The experimental curves are given in ESI,† Fig. S6, while the corresponding real-time plot is given in Fig. 8F. 0.5 mM cysteine was supplemented to E. coli (2.5 × 109 cells per mL), maintained at 37 °C, and the amperometric signal was tracked for a time span of 30 min to 330 min. During the resting time, the electrochemical cell was tightly capped and maintained at 37 °C. The current signal was plotted against time (red lines, Fig. 8F). As per the plot, the response current was increased significantly from 0–60 min, indicating the linear increase of H2S liberation. The limiting signal reached maxima at 60 min, indicating that the maximum H2S production was achieved. The signal however followed a declining trend from 60–180 min, dropped to baseline at 210 min, which indicated that the production of H2S is halted. No obvious current change was noticed from 180 to 240 min. The amount of H2S was quantified by matching the H2S tracking profile with the regression plot derived from H2S quantification carried out in E. coli via a spiking method. After 240 min, H2S production was stimulated again by spiking 0.5 mM cysteine in the reaction mixture and real-time monitoring is resumed. The current was steadily increased again for the next 30 min (240 to 270 min) and dropped after 60 min. However, the control experiments without bacteria or without cysteine have only shown baseline under the same conditions. Next, the production of H2S production was inhibited by a mixture of cysteine and aspartate (H2S inhibitor) to E. coli. The H2S release trend is similar to the stimulation plot but with suppressed signals (green lines, Fig. 8F). About 40% reduction in current signal was observed in the presence of aspartate, which makes sense since a high concentration of aspartate inhibits the production of H2S. In the absence of stimulation, the cells showed no response (blue lines, Fig. 8F). Therefore, a pattern of H2S production is established and any deviation in this triggers an alarm suggesting to check or repair H2S production systems. Our method works on opaque samples without requiring pre-treatments, involving a simpler assay procedure, and depends on an easier electrode preparation strategy. A cell viability test was performed to investigate the biocompatibility of the electrode. The MoS2–ZnCo2O4–ZnCo2O4/CC electrode was transferred to an electrochemical cell containing E. coli cells and incubated for a total of 5 h. Response currents were recorded for each 30 minutes (Fig. 8G). The cell viability of 94% was observed after 5 h of continuous incubation, indicating that the cells were healthy. The use of POPD coating in electrode fabrication is the main reason for the electrode's good biocompatibility. Several previous studies proved that POPD has good anti-biofouling properties in proteinaceous media due to its greater compactness.60
4. Conclusions
In summary, a robust, sensitive carbon cloth electrode modified with MoS2–ZnCo2O4–ZnCo2O4 is developed for real-time in situ sensing of H2S in biological media. The double-layered ZnCo2O4 has better surface properties than single-layered ZnCo2O4 and it can produce dense and uniform MoS2 structures with advanced porous and roughened catalytic sites. MoS2–ZnCo2O4–ZnCo2O4/CC has relatively better electrochemical properties, high electrochemical active area and lower interfacial electron transfer resistance compared to MoS2–ZnCo2O4/CC. In addition, the electrode has excellent H2S sensing ability, anti-poisoning properties, wide linear range (0.01–1000 μM), low detection limit (5 nM), and good selectivity (2.5 fold). The method is practically applicable in human blood, fetal bovine serum, and E. coli cells. MoS2–ZnCo2O4–ZnCo2O4/CC can be used for accurate quantification of endogenous H2S production in bacterial cells, continuously for up to 5 hours. The method would be an effective diagnostic tool for biomedical applications in a point-of-care setting and the use of flexible substrate leaves the possibility of extending its applicability to stretchable biosensors.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by the Ministry of Science and Technology (107-2113-M-027-007- and 108-2221-E-027-063-), Taiwan and King Abdullah University of Science and Technology (KAUST), Saudi Arabia. We also thank the support from Japanese Government MONBUKAGAKUSHO: MEXT Scholarship.References
- G. Rong, S. R. Corrie and H. A. Clark, ACS Sens., 2017, 2, 327–338 CrossRef CAS PubMed.
- G. S. Wilson and R. Gifford, Biosens. Bioelectron., 2005, 20, 2388–2403 CrossRef CAS PubMed.
- M. Asif, W. Haitao, D. Shuang, A. Aziz, G. Zhang, F. Xiao and H. Liu, Sens. Actuators, B, 2017, 239, 243–252 CrossRef CAS.
- H. Yang, Y. Zhang, L. Li, G. Sun, L. Zhang, S. Ge and J. Yu, Biosens. Bioelectron., 2017, 87, 53–58 CrossRef CAS PubMed.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078–10080 CrossRef CAS PubMed.
- H. Kimura, Neurochem. Int., 2013, 63, 492–497 CrossRef CAS PubMed.
- T. Xu, N. Scafa, L.-P. Xu, S. Zhou, K. Abdullah Al-Ghanem, S. Mahboob, B. Fugetsu and X. Zhang, Analyst, 2016, 141, 1185–1195 RSC.
- P. D. Tran, T. V. Tran, M. Orio, S. Torelli, Q. D. Truong, K. Nayuki, Y. Sasaki, S. Y. Chiam, R. Yi and I. Honma, Nat. Mater., 2016, 15, 640–646 CrossRef CAS PubMed.
- O. Yassine, O. Shekhah, A. H. Assen, Y. Belmabkhout, K. N. Salama and M. Eddaoudi, Angew. Chem., Int. Ed., 2016, 55, 15879–15883 CrossRef CAS PubMed.
- E. A. Peter, X. Shen, S. H. Shah, S. Pardue, J. D. Glawe, W. W. Zhang, P. Reddy, N. I. Akkus, J. Varma and C. G. Kevil, J. Am. Heart Assoc., 2013, 2, e000387 Search PubMed.
- M. Dulac, A. Melet and E. Galardon, ACS Sens., 2018, 3, 2138–2144 CrossRef CAS PubMed.
- A. M. O’Mahony, E. J. Dickinson, L. Aldous, C. Hardacre and R. G. Compton, J. Phys. Chem. C, 2009, 113, 10997–11002 CrossRef.
- M. Asif, H. Liu, A. Aziz, H. Wang, Z. Wang, M. Ajmal, F. Xiao and H. Liu, Biosens. Bioelectron., 2017, 97, 352–359 CrossRef CAS PubMed.
- M. Asif, A. Aziz, M. Azeem, Z. Wang, G. Ashraf, F. Xiao, X. Chen and H. Liu, Adv. Colloid Interface Sci., 2018, 262, 21–38 CrossRef CAS PubMed.
- M. D. Brown, J. R. Hall and M. H. Schoenfisch, Anal. Chim. Acta, 2019, 1045, 67–76 CrossRef CAS PubMed.
- N. Wongkaew, M. Simsek, C. Griesche and A. J. Baeumner, Chem. Rev., 2018, 119, 120–194 CrossRef PubMed.
- M. Asif, A. Aziz, Z. Wang, G. Ashraf, J. Wang, H. Luo, X. Chen, F. Xiao and H. Liu, Anal. Chem., 2019, 91, 3912–3920 CrossRef CAS PubMed.
- X. B. Hu, Y. L. Liu, H. W. Zhang, C. Xiao, Y. Qin, H. H. Duo, J. Q. Xu, S. Guo, D. W. Pang and W. H. Huang, ChemElectroChem, 2016, 3, 1998–2002 CrossRef CAS.
- Y. Sharma, N. Sharma, G. Subba Rao and B. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS.
- S. Sahoo and J.-J. Shim, ACS Sustainable Chem. Eng., 2017, 5, 241–251 CrossRef CAS.
- S. G. Surya, S. M. Majhi, D. K. Agarwal, A. A. Lahcen, S. Yuvaraja, K. N. Chappanda and K. N. Salama, J. Mater. Chem. B, 2020, 8, 18–26 RSC.
- W. Luo, X. Hu, Y. Sun and Y. Huang, J. Mater. Chem., 2012, 22, 8916–8921 RSC.
- Y. Gai, Y. Shang, L. Gong, L. Su, L. Hao, F. Dong and J. Li, RSC Adv., 2017, 7, 1038–1044 RSC.
- W. Bai, H. Tong, Z. Gao, S. Yue, S. Xing, S. Dong, L. Shen, J. He, X. Zhang and Y. Liang, J. Mater. Chem. A, 2015, 3, 21891–21898 RSC.
- X. Ge, Z. Li, C. Wang and L. Yin, ACS Appl. Mater. Interfaces, 2015, 7, 26633–26642 CrossRef CAS PubMed.
- I. K. Moon, S. Yoon and J. Oh, Chem. – Eur. J., 2017, 23, 597–604 CrossRef CAS PubMed.
- H. Long, A. Harley-Trochimczyk, S. Cheng, H. Hu, W. S. Chi, A. Rao, C. Carraro, T. Shi, Z. Tang and R. Maboudian, ACS Appl. Mater. Interfaces, 2016, 8, 31764–31771 CrossRef CAS PubMed.
- J. Zhang, S. Cui, Y. Ding, X. Yang, K. Guo and J.-T. Zhao, Biosens. Bioelectron., 2018, 112, 177–185 CrossRef CAS PubMed.
- V. Mani, S. Selvaraj, T.-K. Peng, H.-Y. Lin, N. Jeromiyas, H. Ikeda, Y. Hayakawa, S. Ponnusamy, C. Muthamizhchelvan and S.-T. Huang, ACS Appl. Nano Mater., 2019, 2, 5049–5060 CrossRef CAS.
- J. R. Hall and M. H. Schoenfisch, Anal. Chem., 2018, 90, 5194–5200 CrossRef CAS PubMed.
- M. Pumera and A. H. Loo, TrAC, Trends Anal. Chem., 2014, 61, 49–53 CrossRef CAS.
- Y. Zhang, Z. Hu, Y. Liang, Y. Yang, N. An, Z. Li and H. Wu, J. Mater. Chem. A, 2015, 3, 15057–15067 RSC.
- S. Madhu, A. J. Anthuuvan, S. Ramasamy, P. Manickam, S. Bhansali, P. Nagamony and V. Chinnuswamy, ACS Appl. Electron. Mater., 2020, 2, 499–509 CrossRef CAS.
- L. Shen, Q. Che, H. Li and X. Zhang, Adv. Funct. Mater., 2014, 24, 2630–2637 CrossRef CAS.
- M. Zhen, X. Zhang and L. Liu, RSC Adv., 2016, 6, 43551–43555 RSC.
- Y. Zhou, G. Liu, X. Zhu and Y. Guo, Sens. Actuators, B, 2017, 251, 280–290 CrossRef CAS.
- S. Sahoo and J.-J. Shim, ACS Sustainable Chem. Eng., 2016, 5, 241–251 CrossRef.
- D. Dinda, M. E. Ahmed, S. Mandal, B. Mondal and S. K. Saha, J. Mater. Chem. A, 2016, 4, 15486–15493 RSC.
- J. Cheng, H. Yan, Y. Lu, K. Qiu, X. Hou, J. Xu, L. Han, X. Liu, J.-K. Kim and Y. Luo, J. Mater. Chem. A, 2015, 3, 9769–9776 RSC.
- X.-C. Dong, H. Xu, X.-W. Wang, Y.-X. Huang, M. B. Chan-Park, H. Zhang, L.-H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed.
- W. Zhou, D. Kong, X. Jia, C. Ding, C. Cheng and G. Wen, J. Mater. Chem. A, 2014, 2, 6310–6315 RSC.
- A. Aziz, M. Asif, M. Azeem, G. Ashraf, Z. Wang, F. Xiao and H. Liu, Anal. Chim. Acta, 2019, 1047, 197–207 CrossRef CAS PubMed.
- M. Asif, A. Aziz, H. Wang, Z. Wang, W. Wang, M. Ajmal, F. Xiao, X. Chen and H. Liu, Microchim. Acta, 2019, 186, 61 CrossRef PubMed.
- P. Tamilarasan and S. Ramaprabhu, J. Mater. Chem. A, 2014, 2, 14054–14063 RSC.
- B. Dinesh, K. S. Shalini Devi and A. S. Kumar, J. Electroanal. Chem., 2017, 804, 116–127 CrossRef CAS.
- E. Scavetta, A. Casagrande, I. Gualandi and D. Tonelli, J. Electroanal. Chem., 2014, 722-723, 15–22 CrossRef CAS.
- J. Cheng, Y. Lu, K. Qiu, H. Yan, X. Hou, J. Xu, L. Han, X. Liu, J.-K. Kim and Y. Luo, Phys. Chem. Chem. Phys., 2015, 17, 17016–17022 RSC.
- Y. Fang, J. Pan, J. He, R. Luo, D. Wang, X. Che, K. Bu, W. Zhao, P. Liu and G. Mu, Angew. Chem., Int. Ed., 2018, 130, 1246–1249 CrossRef.
- X.-B. Hu, Y.-L. Liu, W.-J. Wang, H.-W. Zhang, Y. Qin, S. Guo, X.-W. Zhang, L. Fu and W.-H. Huang, Anal. Chem., 2018, 90, 1136–1141 CrossRef CAS PubMed.
- B. Dinesh, K. S. Devi and A. S. Kumar, J. Electroanal. Chem., 2017, 804, 116–127 CrossRef CAS.
- Y. Dilgin, B. Kızılkaya, B. Ertek, N. Eren and D. G. Dilgin, Talanta, 2012, 89, 490–495 CrossRef CAS PubMed.
- K. S. Devi and A. S. Kumar, Analyst, 2018, 143, 3114–3123 RSC.
- I. S. P. Savizi, H.-R. Kariminia, M. Ghadiri and R. Roosta-Azad, Biosens. Bioelectron., 2012, 35, 297–301 CrossRef CAS PubMed.
- M. Asif, A. Aziz, G. Ashraf, Z. Wang, J. Wang, M. Azeem, X. Chen, F. Xiao and H. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 36675–36685 CrossRef CAS PubMed.
- J. A. Bennett, J. E. Pander III and M. A. Neiswonger, J. Electroanal. Chem., 2011, 654, 1–7 CrossRef CAS.
- K. R. Olson, E. R. DeLeon and F. Liu, Nitric oxide, 2014, 41, 11–26 CrossRef CAS PubMed.
- Y.-Q. Dai, D.-M. Zhou and K.-K. Shiu, Electrochim. Acta, 2006, 52, 297–303 CrossRef CAS.
- K. Shatalin, E. Shatalina, A. Mironov and E. Nudler, Science, 2011, 334, 986–990 CrossRef CAS PubMed.
- H. Kimura, Antioxid. Redox Signaling, 2014, 20, 783–793 CrossRef CAS PubMed.
- M. D. Brown and M. H. Schoenfisch, ACS Sens., 2016, 1, 1453–1461 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tb01162b |
| This journal is © The Royal Society of Chemistry 2020 |