Synthetic mycomelanin thin films as emergent bio-inspired interfaces controlling the fate of embryonic stem cells†
Abstract
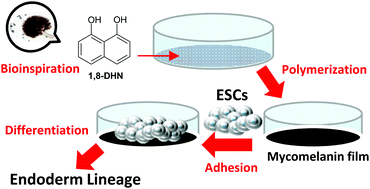
The fungal pathways of melanin synthesis have so far been little considered as a source of bio-inspiration in the field of functional materials, despite the interesting properties exhibited by Ascomycetes melanins from 1,8-dihydroxynaphthalene (1,8-DHN), including the ability to shield organisms from ionizing radiation. Herein, the processing techniques and characterizations of mycomelanin thin films obtained from the solid state polymerization of 1,8-DHN is reported for the first time. Overall, the results highlighted the role of synthetic mycomelanin thin films as a prototype of next generation bioinspired interfaces featuring high structural regularity and ultrasmooth morphology, high robustness against peroxidative bleaching and adhesion under water conditions, good biocompatibility and unprecedented effects in inducing the spontaneous differentiation of embryonic stem cells prevalently towards the endodermal lineages in the absence of added factors. These data open up new avenues towards the applications of this biomaterial in the fields of tissue engineering and regenerative medicine.


 Please wait while we load your content...
Please wait while we load your content...