Bionic composite hydrogel with a hybrid covalent/noncovalent network promoting phenotypic maintenance of hyaline cartilage†
Abstract
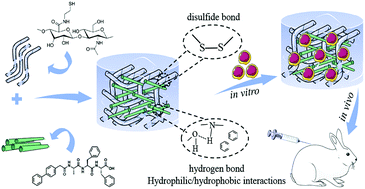
The injectable composite hydrogel based on collagen and hyaluronic acid provided a bionic three-dimensional microenvironment and mimetic natural extracellular matrix (ECM) for the growth of cells in vivo and has been widely researched and developed for cartilage tissue engineering. Here, a novel injectable bionic hydrogel with hybrid covalent/noncovalent network derived from covalent conjugation of HA-SH and noncovalent supramolecular self-assembly of BPAA-AFF-OH short peptide was fabricated to overcome the collagen immunogenicity of animal origin and effectively maintain its biological function. Moreover, through optimizing the network structure and polymer composition, the bionic HS5FFAB5 hydrogel presented a reliable mechanical strength which depended on the highly integrated fiber structure between HA-SH and FFAB-AFF-OH molecules. The results in vitro and in vivo proved that HA-SH could provide a fundamental frame structure, while the supramolecular hydrogels could reinforce this structure via hydrogen bonds and hydrophilic/hydrophobic interactions, and endow bionic hydrogels with more abundant cell adhesion sites. The bionic composite hydrogel could improve the cell adhesion and proliferation when compared to HA-SH hydrogel, and enhanced chondrogenic related gene expression and matrix secretion by three-dimensional co-cultured in vitro and subcutaneous implantation in vivo, which further promoted phenotypic maintenance of hyaline cartilage. This bionic hydrogel with a hybrid covalent/noncovalent network is supposed to have potential application prospects in cartilage regeneration.


 Please wait while we load your content...
Please wait while we load your content...