High-performance non-enzymatic glucose detection: using a conductive Ni-MOF as an electrocatalyst†
Abstract
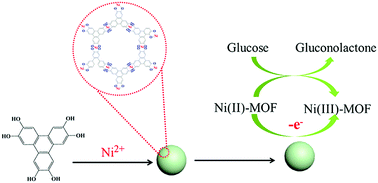
Conductive metal–organic frameworks (MOFs) have been studied extensively in applications like water electrolysis, gas storage, and supercapacitors due to their high conductivity and large pore volume. In this communication, we report the first use of a conductive Ni-MOF as a non-noble-metal catalyst for efficient electro-oxidation of glucose in alkaline electrolyte. As an electrochemical sensor for glucose detection, this Ni-MOF shows a fast response time of less than 3 s, a low detection limit of 0.66 μM (S/N = 3), and a high sensitivity of 21 744 μA mM−1 cm−2. This glucose sensor also displays excellent selectivity, stability and reproducibility, and its application for the detection of glucose in real samples is also demonstrated successfully.
- This article is part of the themed collections: 2020 Journal of Materials Chemistry B most popular articles and Journal of Materials Chemistry B Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...