2D nanostructures beyond graphene: preparation, biocompatibility and biodegradation behaviors
Abstract
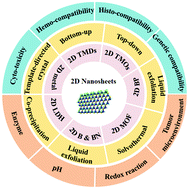
Much attention has been paid to the fabrication of two-dimensional (2D) nanomaterials as therapeutics for nanomedicine in recent years owing to their special physicochemical characteristics. These fascinating physicochemical properties alongside their diverse biomedical applications drive us to give a review of the present endeavors of interest in these 2D nanomaterials. In this review, the up-to-date research advances of the preparation, biocompatibility and biodegradation behaviors of 2D nanomaterials including transition-metal dichalcogenides (TMDs), transition metal oxides (TMOs), black phosphorus (BP) nanosheets, metal–organic frameworks (MOFs), 2D boron (B), boron nitride (BN), layered double hydroxides (LDHs), 2D nanoscale metals, and other kinds of 2D nanomaterials are introduced. The in vitro and in vivo bio-compatibility, including their degradation assessments from the aspects of a redox reaction, enzymes, pH, and the cell environment, etc., of the above categories of 2D nanomaterials are discussed in detail. Finally, the prospects and challenges of the development of 2D nanomaterials aiming for biomedical applications are summarized.
- This article is part of the themed collection: 2020 Journal of Materials Chemistry B most popular articles


 Please wait while we load your content...
Please wait while we load your content...