Adjustable and ultrafast light-cured hyaluronic acid hydrogel: promoting biocompatibility and cell growth†
Abstract
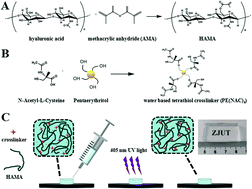
Bio-sourced hydrogels are attractive materials for diagnosing, repairing and improving the function of human tissues and organs. However, their mechanical strength decreases with an increase in water content. Furthermore, it is challenging to mold these hydrogels with high precision, which significantly limits their applications. Herein, we modified a biocompatible and biodegradable material, hyaluronic acid, with methacrylic anhydride and then cured it with a four-arm star structure cross-linking agent under ultraviolet light. The hyaluronic acid hydrogel was finally cured within 15 s with an adjustable cross-linking degree. The results demonstrated that the developed gel maintained good mechanical strength with a water content of 90%, while achieving micropatterns at a precision of 20 μm. The biological experiments showed that it could effectively promote the release of vascular endothelial growth factor (VEGF), which contributed to promoting cell growth, and has favorable biocompatibility. Overall, this hyaluronic acid hydrogel is a promising biomedical material with high forming accuracy, excellent mechanical properties, and favorable biocompatibility, which indicate its potential value in a variety of tissue engineering and biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...