A general strategy for designing NIR-II emissive silk for the in vivo monitoring of an implanted stent model beyond 1500 nm†
Abstract
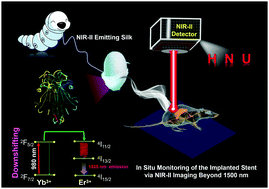
Silk fibroin-based materials spun by silkworms present excellent biocompatible and biodegradable properties, endowing them with broad applications for use in in vivo implanted devices. Therefore, it is highly desirable to explore functionalized silk with additional optical bioimaging abilities for the direct in situ monitoring of the status of implanted devices in vivo. Herein, a new type of silk material with a second near-infrared (NIR-II, 1000–1700 nm) emission is explored for the real-time observation of a biological stent model using a general route of feeding larval silkworms with lanthanide-based NaYF4:Gd3+/Yb3+/Er3+@SiO2 nanocrystals. After being fed lanthanide nanocrystals, the silk spun by silkworms shows efficient NIR-II emission beyond 1500 nm. Moreover, NIR-II bio-imaging guided biological stent model monitoring presents a superior signal-to-noise (S/N) ratio compared to the traditional optical imaging by utilizing the upconversion (UC) region. These findings open up the possibility of designing NIR-II optically functionalized silk materials for highly sensitive and deep-tissue monitoring of the in vivo states of the implanted devices.


 Please wait while we load your content...
Please wait while we load your content...