Nanomaterial-based biosensors for DNA methyltransferase assay
Abstract
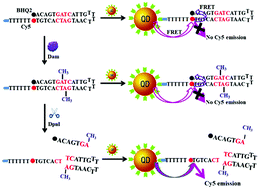
DNA methyltransferases are responsible for catalyzing the methylation of adenine/cytosine residues in specific regions of the genome, and they participate in the establishment of epigenetic modification patterns. Deregulation of DNA methyltransferase activity will disturb DNA methylation systems, leading to the occurrence of various human diseases including cancers. Moreover, DNA methyltransferases may serve as promising therapeutic targets, and DNA methyltransferase inhibitors have been used for disease treatment. Therefore, the detection of DNA methyltransferases and screening of their inhibitors are crucial for both fundamental biomedical research studies and clinical practice. Due to their excellent size-dependent optical, chemical, electronic, and mechanical features, nanomaterials have been widely used as powerful building materials to construct efficient biosensors for DNA methyltransferase assay with high sensitivity and good selectivity. In this review, we summarize the recent progress in the development of nanomaterial-based biosensors for DNA methyltransferase assay including the strategies, features and applications, and highlight the future direction and challenges in this area as well.
- This article is part of the themed collection: Biosensors


 Please wait while we load your content...
Please wait while we load your content...