Conjugation of RTHLVFFARK to human lysozyme creates a potent multifunctional modulator for Cu2+-mediated amyloid β-protein aggregation and cytotoxicity†
Abstract
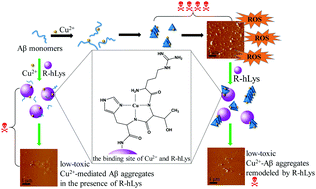
Amyloid β-peptide (Aβ) aggregation induced by metal ions such as Cu2+ has been recognized as a crucial step in the pathogenesis of Alzheimer's disease (AD), so development of multifunctional agents that are able to inhibit Aβ aggregation and modulate Cu2+–Aβ species is considered as a promising strategy for fighting against AD. Our recent work proved that basification of human lysozyme (hLys) is in favor of enhancing the inhibition of Aβ aggregation. Based on the finding, we have herein designed R-hLys, a conjugate of bifunctional alkaline decapeptide (RTHLVFFARK, RK10) coupled onto hLys via reaction with the carboxyl groups on hLys. The design created an even more basic protein than hLys, thus increasing the potency of hLys on inhibiting Aβ fibrillation. Moreover, the RK10 conjugation onto hLys introduced a specific Cu2+-chelator and an additional peptide inhibitor (LVFFARK). Thus, a multifunctional modulator on Cu2+-mediated Aβ aggregation and cytotoxicity was developed. The multifunctional effects of R-hLys were systematically investigated on Aβ aggregation, Cu2+-mediated Aβ aggregation, ROS production, and remodeling mature Aβ species by comparison to its counterparts. The results revealed the following: (1) R-hLys possesses high potency on inhibiting Aβ fibrillogenesis; R-hLys at 1/5 to 1/4 of hLys concentration works similarly to hLys. (2) R-hLys exhibits high performance on inhibiting Cu2+-induced Aβ aggregation and cytotoxicity; it increased the cell viability from 48.9% to 86.1% at an equimolar concentration to Aβ. (3) R-hLys arrests the production of reactive oxygen species catalyzed by Cu2+ or Cu2+–Aβ42 species. (4) R-hLys remodels mature Aβ42 fibrils and Cu2+–Aβ42 aggregates into small amorphous aggregates of lower cytotoxicity; by co-incubation with R-hLys, the cytotoxicities of mature Aβ42 fibrils and Cu2+–Aβ42 aggregates are reduced from 31.4% to 14.2% and 48.1% to 10.4%, respectively. Taken together, R-hLys is a potent multifunctional modulator for inhibiting Aβ/Cu2+-mediated-Aβ aggregation, suppressing ROS production and remodeling mature Aβ42/Cu2+–Aβ42 species.


 Please wait while we load your content...
Please wait while we load your content...