Dual ultrasound-activatable nanodroplets for highly-penetrative and efficient ovarian cancer theranostics†
Abstract
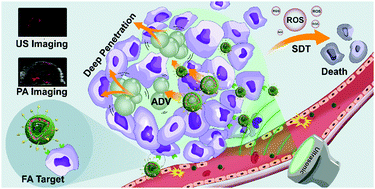
The selective delivery and deep intertumoral penetration of nanosensitizers remain challenging in the fabrication of sonodynamic therapy (SDT) platforms. In this work, we rationally constructed dual ultrasound (US)-activatable nanodroplets (NDs)/nanoliposomes/nanosensitizers with perfluoropentane (PFP) in the core, hematoporphyrin monomethyl ether (HMME) in the phospholipid shell and folate (FA)-conjugated to the surface (collectively termed FA-H@NDs). We aimed to validate the feasibility of these FA-H@NDs for FA receptor (FR)-overexpressed ovarian cancer theranostics. The ND formulations were based on PFP that can undergo acoustic droplet vaporization (ADV) when exposed to US irradiation. The ADV phenomenon disrupts the adjacent vasculature, and the resistance to drug diffusion within the tumor can be decreased, enabling nanosensitizers to more deeply penetrate into the inner tissue far from the intertumoral vasculature. These FA-H@NDs assisted by US irradiation can also induce the production of excess reactive oxygen species (ROS) and consequently trigger tumor cell/tissue apoptosis and necrosis. Furthermore, this therapeutic process can be guided and monitored by US/photoacoustic (PA) dual-modal imaging. This work established a new paradigm for highly efficient ovarian cancer theranostics based on the rational utilization of dual US-activatable NDs.


 Please wait while we load your content...
Please wait while we load your content...