Anisotropic alloying of Re1−xMoxS2 nanosheets to boost the electrochemical hydrogen evolution reaction†
Abstract
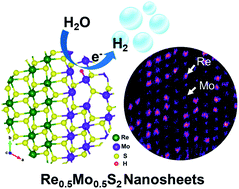
Two-dimensional transition metal dichalcogenides have recently attracted much attention as excellent electrocatalysts for the hydrogen evolution reaction (HER). Herein, Re1−xMoxS2 alloy nanosheets in the entire composition range were synthesized using a hydrothermal reaction. High-resolution scanning transmission electron microscopy revealed anisotropic atomic distribution of the alloy phase, in which the Re and Mo atoms tend to segregate along a crystallographic axis. The phase transition occurs from the triclinic phase (1T′′) ReS2 to the monoclinic phase (1T′) MoS2 at 50% Mo. Re0.5Mo0.5S2 exhibited the highest electrocatalytic HER activity, which was characterized by a current density of 10 mA cm−2 at an overpotential of 98 mV (vs. RHE) and a Tafel slope of 54 mV dec−1 in 0.5 M H2SO4. Extensive calculations using spin-polarized density functional theory showed that the most energetically stable configuration consists of separated MoS2 and ReS2 domains along the b axis, and the 1T′′ → 1T′ phase transition at 50% Mo, which agrees with the experimental results. The Gibbs free energy along the HER pathway indicates that the best performance at Mo 50% is due to the formation of S–H or Mo–H (at S vacancies) on the MoS2 domain.


 Please wait while we load your content...
Please wait while we load your content...