Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Can COFs replace MOFs in flue gas separation? high-throughput computational screening of COFs for CO2/N2 separation†
Omer Faruk
Altundal
,
Cigdem
Altintas
 and
Seda
Keskin
and
Seda
Keskin
 *
*
Department of Chemical and Biological Engineering, Koc University, Rumelifeneri Yolu, Sariyer, 34450, Istanbul, Turkey. E-mail: skeskin@ku.edu.tr; Tel: +90-212-338-1362
First published on 14th July 2020
Abstract
Covalent organic frameworks (COFs) are under study as adsorbent and membrane candidates for gas separation applications. However, experimental testing of all synthesized COF materials as adsorbents and membranes under different operating conditions is not practical. Herein, we used a high-throughput computational screening approach to investigate adsorption- and membrane-based flue gas separation performances of 295 COFs. Adsorption selectivity, working capacity, percent regenerability and adsorbent performance score of COFs were calculated for separation of CO2/N2 mixture for three different cyclic adsorption processes, pressure swing adsorption (PSA), vacuum swing adsorption (VSA) and temperature swing adsorption (TSA). The top performing COFs were identified for each process based on the combination of several metrics. Selectivities of the top COFs were predicted to be greater than those of zeolites and activated carbons. Molecular simulations were performed considering the wet flue gas for the top COF adsorbents and results revealed that most COFs retained their high CO2 selectivities in the presence of water. Using COFs with detailed geometry optimization and high-accuracy partial charges in molecular simulations led to lower selectivities and adsorbent performance scores compared to using experimentally reported COFs with approximate charges. Membrane-based flue gas separation performances of COFs were also studied and most COFs were found to have comparable CO2 permeabilities with metal organic frameworks (MOFs), up to 3.96 × 106 barrer, however their membrane selectivities were lower than MOFs, 0.38–21, due to their large pores and the lack of metal sites in their frameworks. Structure–performance relations revealed that among the COFs we studied, the ones with pore sizes <10 Å, accessible surface areas <4500 m2 g−1 and 0.6 < porosity <0.8 are not only highly selective adsorbents but also CO2 selective membranes.
1. Introduction
Carbon dioxide (CO2) capture from flue gas, CO2/N2 mixture, is environmentally, industrially, and economically important.1,2 Adsorption-based gas separation techniques such as pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and temperature swing adsorption (TSA) have been used for flue gas separation.3,4 An ideal adsorbent should simultaneously offer high selectivity and high working capacity5,6 in addition to being robust under cyclic operation conditions. Activated carbons and zeolites have been utilized as adsorbents but they have limited selectivity and regenerability.7 Therefore, identification of new adsorbent materials offering the best combination of CO2 capacity, CO2/N2 selectivity and regenerability has been a long-standing goal.Metal organic frameworks (MOFs) are porous structures in which nodes consisting of metal clusters are connected through organic linkers.8 The large variety of metal ions and organic linkers enabled the synthesis of many different materials with tunable chemistries, low densities (0.2 g cm−3), large surface areas (>6000 m2 g−1), various pore sizes and high porosities (0.3–0.9).9,10 MOFs have been considered as promising materials for adsorption-based CO2/N2 separation due to these structural and chemical aspects.11,12 Several computational studies used Grand Canonical Monte Carlo (GCMC) simulations to predict CO2/N2 separation performances of various MOFs.13,14 For example, early studies showed CuBTC had a slightly higher CO2/N2 selectivity (∼33) than zeolite MFI (∼27) for separation of CO2/N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15/85 mixture at PSA condition.13 Krishna and van Baten15 showed that MgMOF-74 has a similar selectivity to that of NaX (∼200) with a higher working capacity (∼3 mol kg−1) than that of NaX (∼2 mol kg−1) for CO2/N2
15/85 mixture at PSA condition.13 Krishna and van Baten15 showed that MgMOF-74 has a similar selectivity to that of NaX (∼200) with a higher working capacity (∼3 mol kg−1) than that of NaX (∼2 mol kg−1) for CO2/N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15/85 mixture at VSA condition at 300 K. With these, the promise of MOFs in adsorption-based CO2/N2 separation has been proven.16–18
15/85 mixture at VSA condition at 300 K. With these, the promise of MOFs in adsorption-based CO2/N2 separation has been proven.16–18
Due to the acceleration in the synthesis of MOFs, experimental investigation of MOFs for flue gas separation has become challenging. High-throughput computational screening methods have been very useful for efficiently screening large numbers of MOFs to guide experimental studies to more promising MOFs. The first high-throughput screening of MOFs for CO2/N2 separation was carried out by studying 489 MOFs and two MOFs were highlighted with their high CO2/N2 mixture selectivities (269 and 197) at 1 bar, 303 K.19 Wu et al.20 studied 105 different MOFs and showed that higher CO2/N2 selectivities (>120) can be obtained at 1 bar, 298 K if the isosteric heats of adsorption of CO2 and N2 is highly different in MOFs having low porosities. Wilmer et al.21 examined flue gas separation potentials of 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hypothetical MOFs at VSA condition at 298 K and showed that the best materials have pore sizes <5 Å, porosities in the range of 0.3–0.4. Qiao et al.22 screened 4764 MOFs23 for flue gas separation at VSA condition and showed that most of the promising MOFs have lanthanides. Our group recently computed several adsorbent performance evaluation metrics of 3816 MOFs for separation of CO2/N2 mixture at VSA conditions and reported that the best MOF candidates have 3.8 Å < pore limiting diameter <5 Å and surface area <1000 m2 g−1.10 Molecular simulations were also used to investigate membrane-based flue gas separation potential of MOFs. For example, Krishna and van Baten24 screened a large number of MOF, zeolitic imidazolate framework (ZIF), and zeolite membranes using Configurational-Bias Monte Carlo (CBMC) and Molecular Dynamics (MD) simulations. They reported that CO2/N2 selectivity (∼20) and CO2 permeability (∼8 × 105 barrer) of Mg-MOF-74 was higher than those of many zeolites (DDR, ERI, MFI, TSC, and FAU). Watanabe and Sholl25 calculated CO2 permeabilities and CO2/N2 selectivities of 179 MOFs at infinite dilution and reported that MOF membranes offer superior CO2 permeabilities (104 barrer) and CO2/N2 selectivities (>100) than polymeric membranes. Our group recently studied 3806 MOF membranes at infinite dilution and the top 15 MOFs having high CO2/N2 selectivity in the range of 16–820 at 1 bar, 298 K were identified.26 All these works revealed the high potential of MOF adsorbents and membranes for CO2/N2 separation. However, MOFs offering the highest CO2 separation performances under dry flue gas condition may suffer from a decrease in CO2 selectivity and/or a stability issue under humid environment and therefore, the search for robust MOFs and MOF-like materials is still continuing.27–32
000 hypothetical MOFs at VSA condition at 298 K and showed that the best materials have pore sizes <5 Å, porosities in the range of 0.3–0.4. Qiao et al.22 screened 4764 MOFs23 for flue gas separation at VSA condition and showed that most of the promising MOFs have lanthanides. Our group recently computed several adsorbent performance evaluation metrics of 3816 MOFs for separation of CO2/N2 mixture at VSA conditions and reported that the best MOF candidates have 3.8 Å < pore limiting diameter <5 Å and surface area <1000 m2 g−1.10 Molecular simulations were also used to investigate membrane-based flue gas separation potential of MOFs. For example, Krishna and van Baten24 screened a large number of MOF, zeolitic imidazolate framework (ZIF), and zeolite membranes using Configurational-Bias Monte Carlo (CBMC) and Molecular Dynamics (MD) simulations. They reported that CO2/N2 selectivity (∼20) and CO2 permeability (∼8 × 105 barrer) of Mg-MOF-74 was higher than those of many zeolites (DDR, ERI, MFI, TSC, and FAU). Watanabe and Sholl25 calculated CO2 permeabilities and CO2/N2 selectivities of 179 MOFs at infinite dilution and reported that MOF membranes offer superior CO2 permeabilities (104 barrer) and CO2/N2 selectivities (>100) than polymeric membranes. Our group recently studied 3806 MOF membranes at infinite dilution and the top 15 MOFs having high CO2/N2 selectivity in the range of 16–820 at 1 bar, 298 K were identified.26 All these works revealed the high potential of MOF adsorbents and membranes for CO2/N2 separation. However, MOFs offering the highest CO2 separation performances under dry flue gas condition may suffer from a decrease in CO2 selectivity and/or a stability issue under humid environment and therefore, the search for robust MOFs and MOF-like materials is still continuing.27–32
Covalent organic frameworks (COFs) are a class of porous materials containing light elements (hydrogen, boron, carbon, nitrogen, oxygen, silicon) linked by strong covalent bonds, like in diamond.30,33–37 Since COFs are light materials and have strong covalent bonds, they show low density, good thermal and chemical stability, and permanent porosity.37 Compared to MOFs, 3-dimensional COFs can offer a higher fraction of accessible surface area for gas adsorption.38 Tong et al.39 computationally studied 46 COFs for separation of CH4/H2, CO2/CH4, and CO2/H2 mixtures at PSA condition and showed that COFs offer better CO2 working capacities (>3 mol kg−1) than many conventional zeolites and common MOFs. A computation-ready experimental COF database (CoRE COF) consisting of 187 solvent-free COFs was created,40 later expanded41 and finally reported to include 309 COFs which facilitated high-throughput computational screening of these materials.42 Using this database, 290 functionalized COFs were designed and 137 of these COFs were predicted to perform better than conventional polymers for membrane-based CO2/CH4 separation.42 Ongari et al.43 recently reported a publicly available clean, uniform and refined with automatic tracking from experimental COF database (CURATED COFs) consisting of 324 optimized COFs with high-quality partial charges. Smit's group44 predicted CO2 parasitic energy of the CURATED COFs43 and hypothetical COFs using molecular simulations and showed that many COFs have lower energy than that of traditional amine scrubbing process. As can be seen from this literature summary, we have limited information on the adsorption and membrane-based CO2/N2 separation performances of COFs and the high-throughput computational screening of COFs is essential to compare their adsorbent and membrane performance evaluation metrics with those of MOFs.
Motivated from this, in this work, we searched the answer to the following question: can COFs replace or at least compete with MOFs as adsorbent and membrane materials in flue gas separation processes? We first performed a high-throughput computational screening study on CoRE COF database to unlock both adsorption-based and membrane-based CO2/N2 separation potentials of all experimentally synthesized COFs. CO2/N2 mixture adsorption in COFs was computed using GCMC simulations and results were used to evaluate selectivity, working capacity, adsorbent performance score and regenerability of COF adsorbents at three different cyclic adsorption conditions, PSA, VSA, and TSA. Efficiencies of these three processes were then compared using adsorbent performance evaluation metrics to select the most promising COFs for each operating condition. The effect of humidity on the flue gas separation performance of the most promising COF adsorbents was also investigated by performing simulations for adsorption-based CO2/N2/H2O mixture separation. We also utilized CURATED COF database for molecular simulations and compared the results with those of CoRE COF database to understand how structural curations on the experimentally reported COFs affect their predicted CO2/N2 separation performances. We finally assessed membrane-based CO2/N2 mixture separation performances of COFs by combining the results of GCMC and MD simulations and compared gas permeabilities and selectivities of COF membranes with those of polymers. The large-scale screening of COFs performed in this work allowed us to elucidate the structure–performance relations that can help to describe the structural features leading to high-performance COF adsorbents and membranes. All these results will (i) provide the first comparison for the flue gas separation performances of COFs, MOFs, and established adsorbents and membranes including zeolites and polymers, (ii) guide the future studies to the most promising COFs identified from high-throughput screening and (iii) contribute to the intuition at the molecular scale for the design and advancement of new COF adsorbents and membranes with more efficient CO2 separation potentials.
2. Computational details
2.1. COF database
We used the most recent CoRE COF database in the literature consisting of 309 COF structures.42 Structural properties of COFs; pore limiting diameter (PLD), largest cavity diameter (LCD), porosity (ϕ), density (ρ) and accessible surface area (ASA) were computed employing Zeo++ software.45 A probe diameter of 3.72 Å which corresponds to the kinetic diameter of N2 molecule was used for surface area determination whereas a probe with zero diameter was used for the pore volume calculations. CoRE COF database was then refined to only include the structures with non-null ASA so that both gases can adsorb in the pores. Consequently, 295 COFs remained in the database offering a diverse range of structural properties. Porosities of these COFs were computed to be between 0.44–0.96 while their PLDs and ASAs were calculated to be in the range of 4.19–44.50 Å and 245–8561 m2 g−1, respectively. We note that atomistic coordinates of HAT-NTBA-COF was corrected by using the coordinates taken from its experimental synthesis paper46 following the warning in the literature.43 To examine the effect of structural curations performed on the experimental CoRE COF database, we also studied 23 materials from the CURATED COF database.2.2. Molecular simulations
We used the high-throughput computational screening approach that we introduced and used for MOFs in our previous works10,47 where we showed that the results of our molecular simulations were in accordance with experimental CO2 and N2 uptakes of MOFs. To compute the CO2/N2 mixture adsorption data of COFs, GCMC simulations were performed with RASPA 2.35 simulation code.48 We specified the composition of CO2/N2 as 15/85 to represent dry flue gas. GCMC simulations were performed at three different operating conditions; VSA, PSA, and TSA. For VSA and PSA processes, temperature was fixed at 298 K while adsorption (desorption) pressure was set as 1 (0.1) bar at VSA and 10 (1) bar at PSA. For TSA process, pressure was set as 1 bar and temperatures for adsorption and desorption were set as 298 K and 393 K, respectively.49 Peng–Robinson equation of state was used for pressure to fugacity conversion. Non-bonded interactions were defined with Lennard-Jones (LJ) potential and a cut-off distance of 14 Å was specified for truncation of these interactions. The number of unit cells in the simulation box was adjusted according to the cut-off distance. Electrostatic interactions between adsorbate–adsorbate and adsorbate-COF atoms were considered due to the quadrupolar moments of CO2 and N2. Partial atomic charges of COF atoms were assigned via the charge equilibration method (Qeq).50 The partial charges of atoms in a COF, COF-SDU1, did not converge using the Qeq in the RASPA and assigned by the Qeq implemented in Materials Studio.51 The Ewald summation was implemented to incorporate long–range interactions.52 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles were set for initialization and 20
000 cycles were set for initialization and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles were set for taking ensemble averages in GCMC simulations. Potential parameters for CO2 molecule were taken from the TraPPE53 force field. For CO2 molecule, a three-site rigid model with LJ 12–6 potential was used where the partial point charges were set at the center of each site.54 Similarly, for N2 molecule, a three-site model was used with partial charges at the center of mass.55 DREIDING56 was used for the potential parameters of COF atoms since this force field was shown to give a good agreement with the experimentally reported single-component CO2 and CH4 adsorption isotherms of COFs in the literature.42 We also showed the good agreement between our simulations using DREIDING and experimentally measured CO2 adsorption isotherms of several COFs in Fig. S1 of the ESI.† To understand the effect of humidity, CO2/N2/H2O
000 cycles were set for taking ensemble averages in GCMC simulations. Potential parameters for CO2 molecule were taken from the TraPPE53 force field. For CO2 molecule, a three-site rigid model with LJ 12–6 potential was used where the partial point charges were set at the center of each site.54 Similarly, for N2 molecule, a three-site model was used with partial charges at the center of mass.55 DREIDING56 was used for the potential parameters of COF atoms since this force field was shown to give a good agreement with the experimentally reported single-component CO2 and CH4 adsorption isotherms of COFs in the literature.42 We also showed the good agreement between our simulations using DREIDING and experimentally measured CO2 adsorption isotherms of several COFs in Fig. S1 of the ESI.† To understand the effect of humidity, CO2/N2/H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15/82/3 ternary mixture simulations were performed. The partial pressure of H2O was kept at 3 kPa, which is 70% of the vapor pressure of H2O in TIP4P57,58 model at 298 K (4.3 kPa). The Henry's constants of H2O in COFs were also calculated to quantify the affinity of COFs to water at 298 K by conducting simulations with only Widom particle insertion move in RASPA using 105 cycles.59 Using the results of GCMC simulations, adsorption selectivity, working capacity, adsorbent performance score and percent regenerability were computed as shown in Table 1 to assess the performances of COF adsorbents for gas separation. CO2 and N2 self-diffusivities were calculated using MD simulations which were performed for 5 × 106 cycles in the NVT ensemble with a time step of 1 fs. 106 cycles were set both for equilibration and initialization of NVT-MD simulations in which the Nosé–Hoover60 thermostat was utilized. Self-diffusivities of gases were calculated from the slope of their mean square displacements according to the Einstein's relation.61 Gas permeabilities and selectivities of COF membranes were predicted by combining the results of GCMC and MD simulations as shown in Table 1. Two feed pressures, 1 bar and 10 bar, were used while the permeate pressure was set as vacuum. Gas loadings obtained from GCMC simulations at 1 bar and 10 bar were used as the input of MD simulations.62 Atomic coordinates of COF atoms were fixed during calculations to save computational time. Since pore sizes of COFs studied in this work are large enough with respect to the kinetic diameters of both gases, flexibility was expected to not considerably effect our results as we discussed before.63
15/82/3 ternary mixture simulations were performed. The partial pressure of H2O was kept at 3 kPa, which is 70% of the vapor pressure of H2O in TIP4P57,58 model at 298 K (4.3 kPa). The Henry's constants of H2O in COFs were also calculated to quantify the affinity of COFs to water at 298 K by conducting simulations with only Widom particle insertion move in RASPA using 105 cycles.59 Using the results of GCMC simulations, adsorption selectivity, working capacity, adsorbent performance score and percent regenerability were computed as shown in Table 1 to assess the performances of COF adsorbents for gas separation. CO2 and N2 self-diffusivities were calculated using MD simulations which were performed for 5 × 106 cycles in the NVT ensemble with a time step of 1 fs. 106 cycles were set both for equilibration and initialization of NVT-MD simulations in which the Nosé–Hoover60 thermostat was utilized. Self-diffusivities of gases were calculated from the slope of their mean square displacements according to the Einstein's relation.61 Gas permeabilities and selectivities of COF membranes were predicted by combining the results of GCMC and MD simulations as shown in Table 1. Two feed pressures, 1 bar and 10 bar, were used while the permeate pressure was set as vacuum. Gas loadings obtained from GCMC simulations at 1 bar and 10 bar were used as the input of MD simulations.62 Atomic coordinates of COF atoms were fixed during calculations to save computational time. Since pore sizes of COFs studied in this work are large enough with respect to the kinetic diameters of both gases, flexibility was expected to not considerably effect our results as we discussed before.63
| Metrics | Formula |
|---|---|
| a i: gas species, CO2 or N2. Nads (mol kg−1): gas uptake at adsorption conditions. Ndes (mol kg−1): gas uptake at desorption conditions. y: composition of the gas species in the bulk phase. f (Pa): partial pressure of gas species in the mixture. c (mol m−3): gas concentration obtained from GCMC simulations. D (m2 s−1): self-diffusivity of gas obtained from MD simulations. 1 barrer = 3.348 × 10−16 mol × m (m2 × s × Pa)−1. | |
| Adsorption selectivity |
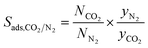
|
| Working capacity (mol kg−1) | ΔN = Nads,CO2 − Ndes,CO2 |
| Adsorbent performance score (mol kg−1) | APS = Sads,CO2/N2 × ΔNCO2 |
| Percent regenerability |
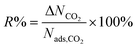
|
| Diffusion selectivity |
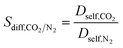
|
| Membrane selectivity | S mem,CO2/N2 = Sads,CO2/N2 × Sdiff,CO2/N2 |
| Permeability (barrer) |
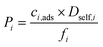
|
3. Result and discussion
3.1 Adsorption-based separation performances of COFs
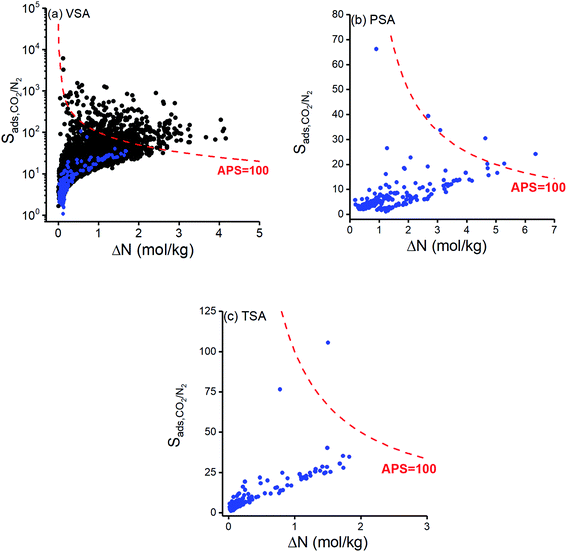
We first examined adsorption-based CO2/N2 separation potentials of 295 COFs. Fig. 1 represents CO2/N2 selectivity of COFs as a function of their CO2 working capacity at (a) VSA (b) PSA and (c) TSA conditions. CO2/N2 selectivity and CO2 working capacity of 3808 MOFs which were previously computed at VSA condition10 were also shown in Fig. 1(a). CO2 selectivities were computed to be between 1–105 and 2–6107 for COFs and MOFs, respectively, whereas CO2 working capacities were calculated to be between 0.02–1.69 mol kg−1 and 0.01–4.16 mol kg−1 for COFs and MOFs, respectively. This comparison shows that COFs have lower CO2/N2 selectivities than most of the MOFs at VSA condition which can be explained with larger pore sizes of COFs compared to MOFs and the absence of metal sites in COFs. Only 5% of MOFs shown in Fig. 1(a) have LCDs > 15 Å while 70% of COFs have LCDs > 15 Å. Large pores of COFs allow both gas molecules to be adsorbed in the framework, reducing the selective adsorption property of materials. In addition to this, MOFs have metal sites leading to strong coulombic interactions between the framework atoms and CO2 molecules.64 Most COFs do not have those metal sites. For example, in the whole set of 295 COFs that we examined, only 24 COFs have metal sites with relatively high partial charges (8 with Cu sites, 6 with Ni sites, 5 with Zn sites, 4 with Co sites and 1 with Li sites). As a result of this, most COFs have weaker interactions with the adsorbate molecules compared to MOFs. Detailed information about the distribution of elements in COFs that we examined in this work can be seen in Fig. S2.† Performances of the adsorbents are generally assessed by considering both selectivity and working capacity because a promising adsorbent should offer both high working capacity and high selectivity for an efficient and economic gas separation process. We used the adsorbent performance score (APS), which is the multiplication of adsorption selectivity and working capacity as shown in Table 1, to appraise the performances of COFs for CO2/N2 separation. We arbitrarily set APS to 100 mol kg−1, as shown with the red curves in Fig. 1, to identify good performing materials. Fig. 1(a) shows that COFs have relatively lower APSs than MOFs mainly due to their lower selectivities at VSA condition. Even the COF with the highest APS value (ICOF-2, APS: 59.59 mol kg−1) cannot compete with MOFs, of which the APS can be higher than 2000 mol kg−1. We, therefore, concluded that MOFs perform better than COFs for adsorption-based separation of dry flue gas at VSA condition.Calculated CO2 selectivities of COFs at PSA and TSA conditions were between 1–66 and 1–105, respectively, whereas CO2 working capacities were in the range of 0.18–6.36 mol kg−1 and 0.02–1.83 mol kg−1 as shown in Fig. 1(b) and (c), respectively. APSs of MOFs at PSA (TSA) condition were calculated to vary between 0.65 and 153.64 mol kg−1 (0.04 and 158.79 mol kg−1). Although selectivities of COFs at PSA were computed to be lower than those calculated at VSA and TSA conditions, APSs of COFs were found to be the highest at PSA because of the high CO2 working capacities. None of the COFs was able to surpass APS = 100 mol kg−1 curve at VSA condition while 5 COFs were identified to have APSs > 100 mol kg−1 at PSA condition as shown in Fig. 1(b), and only one COF exceeds APS = 100 mol kg−1 target at TSA condition as shown in Fig. 1(c). The COF that exceeds the APS target in TSA (ICOF-2) has Li+ cations which significantly contributed to coulombic interactions between COF atoms and CO2 molecules. Our simulation results showed that electrostatic interactions were responsible for 89% of the total interaction energy between this COF and guest molecules. Since the quadrupole moment of N2 (4.65 × 10−40 C × m2) is smaller than that of CO2 (14.27 × 10−40 C × m2),65 electrostatic interactions become more pronounced for CO2 than N2.
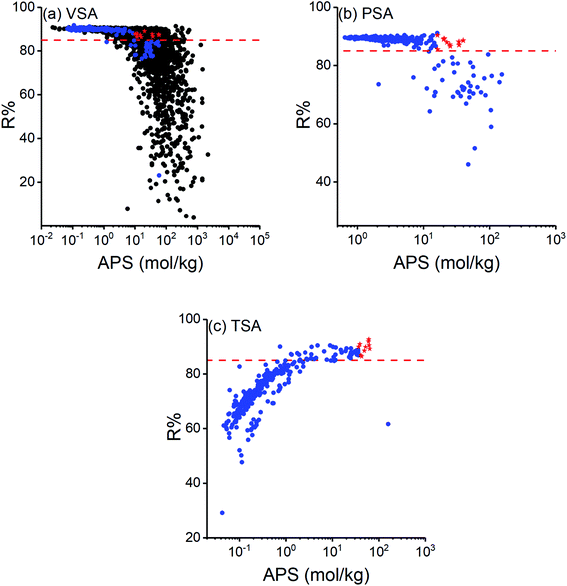
Regenerability (R%) is an essential factor when assessing the practical usage of adsorbents for cyclic processes and it should be taken into account when selecting the most promising materials for cyclic VSA, PSA and TSA processes.66 This is because adsorbents offering high selectivities generally suffer from low R% values. Calculated R% of COFs are shown as a function of their APSs in Fig. 2 where the red dashed line represents R% = 85 which we set as the minimum acceptable R%. High R% of COFs were generally obtained at VSA and PSA conditions as shown in Fig. 2(a) and (b), respectively, and COFs were found to have higher R% than most MOFs as shown in Fig. 2(a). More than 80% of COFs were computed to have R% > 85% at VSA and PSA conditions while this ratio was 18% at TSA condition. At VSA and PSA conditions R% values were generally high but tend to decrease as APS increases, which was previously observed for MOFs in adsorption-based CH4/H2 separation.66 This indicates that many COFs with high APSs suffer from low R%. For example, ICOF-2 would be a promising material at all cyclic adsorption process conditions we studied in this work if we only focused on selectivity and APS. However, R% was calculated to be very low for this COF (23.12% for VSA, 45.98% for PSA and 61.60% for TSA), which makes it practically unusable under these operating conditions.
Fig. 2(c) shows that R% values computed for TSA condition were generally lower than the ones obtained at VSA and PSA conditions. Also, interestingly, these R% values showed a different trend than those computed for VSA and PSA conditions and increased as a function of APS. When we consider the calculation of APS (Sads × ΔN), R% (ΔN/Nads), and ΔN (Nads − Ndes), only the amount of gas at the desorption condition (Ndes) is different for VSA and TSA processes as both share the same adsorption temperature and pressure (1 bar, 298 K). Therefore, a higher R% at either of these conditions indicates that a higher amount of CO2 is desorbed. At the desorption condition of TSA (1 bar, 393 K), adsorbed gas molecules did not desorb as much as at desorption condition of VSA (0.1 bar, 298 K) for most of the COFs, especially the ones with APS <10 mol kg−1. This is shown in Fig. S3† where higher Ndes indicates that less amount of gas is desorbed. Based on this, we can conclude that pressure have a more pronounced effect on gas desorption than temperature for most COFs.
We aimed to identify the most promising adsorbent candidates in each process by specifically focusing on COFs with R% > 85% and ranking them based on their APSs. COFs with R% > 85% were found to have APSs in the range of 0.07–58.95 mol kg−1 at VSA, 0.65–39.70 mol kg−1 at PSA and 0.74–63.43 mol kg−1 at TSA conditions. 10 COFs with the highest APSs were selected for each operating condition as the most promising materials and shown by red stars in Fig. 2. Among these, COF-42-gra, FLT-COF-1-staggered, COF-JLU3 and NPN-2 were the common top materials for VSA and TSA processes. Calculated adsorbent performance metrics and the names of the top COFs are given in Table 2 along with the selectivities and working capacities of some commercial adsorbents used in the industry to compare flue gas separation performances of different materials. Unit cell representations of the most promising COFs for each process condition are given in Fig. S4.†Table 2 shows that many of the promising COF adsorbents have higher CO2/N2 selectivities than commercial adsorbents while having comparable working capacities to them. For example, zeolite 13X provided a selectivity of 17 at 1 bar, 298 K and working capacity of 2.30 mol kg−1 at VSA conditions. The selectivities and working capacities of the top COFs of VSA condition were in the range of 14–40 and 0.58–1.67 mol kg−1, respectively. This indicates that COFs have the potential to replace zeolites in adsorption-based CO2/N2 separation. The top COFs identified for TSA process have higher APSs compared to the top COFs identified for VSA and PSA conditions, suggesting that when APS and R% were both considered, TSA can be the best process option for adsorption-based flue gas separation using COFs. Most COFs have high thermal stability37 but to ensure if the top COFs that we identified for TSA process can be robust under temperature changes, we confirmed the thermal stabilities of these top COFs by checking their experimental synthesis papers. For instance, one of the promising COFs, 3D-COOH-COF, was reported to be thermally stable up to 723 K (ref. 67) and another promising COF, COF-42-gra, was thermally stable up to 553 K.68 These temperatures are much higher than the desorption temperature of the TSA condition, 393 K, that we considered in this work.
| Top COFs | S ads | ΔN (mol kg−1) | APS (mol kg−1) | R% |
|---|---|---|---|---|
| a Calculated using Ideal adsorbed solution theory (IAST).87 b This COF was reported to lose its crystal structure upon solvent removal.88 c This COF was reported without Co atoms in databases.89 | ||||
| Vacuum swing adsorption (VSA) | ||||
| COF-42-gra | 40.20 | 1.41 | 56.70 | 87.54 |
| COF-43-gra | 24.75 | 0.81 | 20.11 | 89.07 |
| COF-JLU3 | 24.91 | 1.43 | 35.65 | 87.74 |
| CuP-TFPh COF | 16.78 | 0.94 | 15.71 | 86.35 |
| FLT-COF-1 staggered | 35.28 | 1.67 | 58.95 | 87.30 |
| NPN-2 | 25.26 | 1.47 | 37.11 | 86.64 |
| Ph-AnCD-COF | 14.03 | 0.82 | 11.51 | 86.51 |
| PyTTA-BFBIm-iCOF | 16.96 | 0.88 | 14.87 | 87.33 |
| TpMA | 21.77 | 0.47 | 10.15 | 87.83 |
| Tp-Por COF-AB | 19.98 | 0.58 | 11.58 | 87.98 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Pressure swing adsorption (PSA) | ||||
| COF-102 | 6.16 | 2.63 | 16.19 | 90.38 |
| COF-300 | 9.52 | 2.65 | 25.28 | 86.63 |
| COF-6 | 10.84 | 1.86 | 20.14 | 89.01 |
| COF-DL229-6 fold | 10.40 | 2.40 | 24.92 | 87.21 |
| EB-COF:F | 18.52 | 1.86 | 34.38 | 87.08 |
| SIOC-COF-4-AB | 7.17 | 2.27 | 16.29 | 85.53 |
| TPE-COF-I | 12.03 | 3.30 | 39.70 | 88.63 |
| TPE-Ph COF | 8.07 | 2.79 | 22.52 | 87.95 |
| TpPa-1-F2 | 12.83 | 1.60 | 20.52 | 89.28 |
| TpPa-F4 | 18.17 | 1.88 | 34.21 | 88.21 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Temperature swing adsorption (TSA) | ||||
| 3D-COOH-COF | 76.52 | 0.78 | 59.58 | 91.89 |
| COF-42-gra | 40.20 | 1.49 | 60.05 | 92.70 |
| COF-JLU3 | 24.91 | 1.47 | 36.55 | 89.97 |
| CoPc-PorDBAc | 27.89 | 1.74 | 48.48 | 88.48 |
| FLT-COF-1 staggered | 35.28 | 1.73 | 61.11 | 90.51 |
| NPN-1b | 30.45 | 1.68 | 51.27 | 89.90 |
| NPN-2 | 25.26 | 1.54 | 38.95 | 90.94 |
| PI-COF | 34.71 | 1.83 | 63.43 | 89.23 |
| POR-COF | 26.09 | 1.39 | 36.18 | 86.82 |
| TThPP | 28.51 | 1.42 | 40.47 | 86.82 |
| Adsorbents | Conventional adsorbents | Ref. | |
|---|---|---|---|
| S ads | ΔN (mol kg−1) | ||
| NaX | 226.00 (IASTa, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, 298 K) 85, 298 K) |
1.47 (1–0.1 bar) | 81 |
| NaY | 14.84 (ideal, 1 bar, 303 K) | 1.87 (1–0.1 bar) | 82 |
| Zeolite 13X | 17.45 (ideal, 1 bar, 298 K) | 2.30 (1–0.1 bar) | 83 |
| CHA | 27.00 (ideal, 1 bar, 300 K) | 3.60 (10–1 bar) | 84 |
| Hβ | 11.33 (ideal, 1 bar, 300 K) | 1.43 (1–0.1 bar) | 85 |
| Activated carbon | 8.86 (ideal, 1 bar, 293 K) | 2.58 (1–0.1 bar) | 86 |
After identifying the top COF adsorbents, we investigated the separation performances of these materials under humid conditions by performing GCMC simulations for ternary CO2/N2/H2O mixture. We first calculated the Henry's constants of water (KH, water) for these COFs and found that KH, water values of 15 of the 26 distinct top COFs (top 10 COFs were identified for each process and 4 top materials were common for VSA and TSA) were smaller than that of ZIF-8 (6.21 × 10−6 mol kg−1 Pa−1), which is known to be a hydrophobic MOF.69,70 Therefore, it was seen that most of the top COFs we studied were also hydrophobic. Then, we performed adsorption simulations for wet flue gas, i.e. CO2/N2/H2O mixture. Calculated CO2 selectivities of hydrophobic COFs for wet flue gas were found to be only slightly lower (6–56 and 7–61 at 1 and 10 bar, respectively) than those computed for dry flue gas (7–77 and 7–66 at 1 and 10 bar, respectively). However, COFs having higher KH, water values were found to have significantly lower CO2 selectivities for wet flue gas compared to those calculated for dry flue gas. For example, CO2 selectivities of CuP-TFPh COF (KH, water = 3.12 × 10−4 mol kg−1 Pa−1) decreased from 17 to 1 at 1 bar and from 13 to 3 at 10 bar when ternary CO2/N2/H2O mixture was considered. We, therefore, concluded that humidity decreases the CO2 selectivities of hydrophilic COFs in agreement with the results obtained when MOFs were studied for wet flue gas separation,71 but hydrophobic COFs retained their high CO2 selectivities in the presence of humidity.
3.2 Effects of structural curations on the simulated performances of COFs
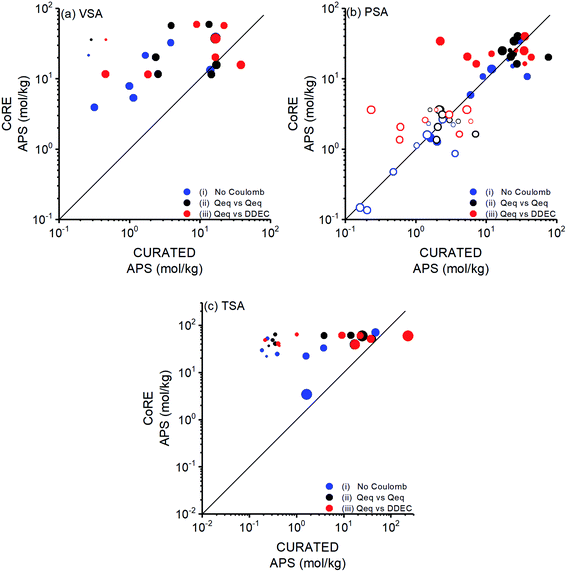
We aimed to compare performances of COFs for CO2/N2 separation with those of MOFs. Therefore, COF structures in this work were taken from the CoRE COF database and used without further optimization as we previously did in our large-scale screening of the MOF database.10 While we were working on CoRE COFs, CURATED COF database, geometry optimized by considering symmetry and stacking of structures with a more detailed procedure than that of CoRE COFs,40,43 was reported. The main input of our molecular simulations is the crystal structures of COFs. Therefore, we aimed to understand how structural differences due to different optimization methods will affect simulation results and hence predicted flue gas separation performances of COFs. To investigate this, we focused on the top COFs identified from the CoRE COF database, computed their adsorbent performance metrics, and compared them with those computed for CURATED COFs. 9 (8) of the top COFs identified for PSA (VSA) process were available in CURATED COF database and all top performing COFs identified for TSA were available in the CURATED COF database.We arranged the size of the data points in Fig. 3 according to the ratio of a structural property (ASA, ρ, and ϕ) of a CURATED COF to that of a CoRE COF and related the change in the calculated performance metrics to the change in the COF structure. The most significant change was observed for ASA of COFs. Fig. 3 shows the change in calculated APSs of COFs at VSA, PSA and TSA conditions according to ASACURATED/ASACoRE. The minimum, average, and maximum ratio of ASAs of CURATED and CoRE COFs (ASACURATED/ASACoRE) were calculated as 0.30, 0.69, and 1.16, respectively. If a COF is shown with a clearly different symbol size that means its ASA was significantly different when it was taken from CoRE COFs or CURATED COFs. APSs of COFs significantly changed especially when the structure optimization led to very different ASAs as presented with the symbol size in Fig. 3. It is important to note that the CURATED COFs do not only differ from CoRE COFs because of the detailed geometry optimization but they were also reported with high accuracy partial charges. In order to isolate the charge effect, we compared performance metrics under three different cases in Fig. 3: (i) when the coulombic interactions between adsorbents and gas molecules were neglected (blue points), (ii) when the charges of both COFs were assigned using the Qeq method (black points), and (iii) when the charges of CoRE COFs were assigned using the Qeq method while the charges of CURATED COFs were taken as the density-derived electrostatic and chemical charges-DDEC (red points). With case (i) and (ii) we examined only the effect of structural discrepancies on performance metrics while with case (iii) combined effect of different structural properties and different partial charges on performance metrics was investigated. Fig. 3(a) shows that APSs of most COFs significantly change for all three cases at VSA conditions. The coefficients of determination (R2) between APSs of CoRE and CURATED COFs were calculated as 0.35 for case (i), 0.10 for case (ii) and 0.07 for case (iii) at VSA condition. In case (i), only the effect of geometry optimization was considered and the highest changes in APSs were observed for the COFs with the highest ASACURATED/ASACoRE values. This can be explained with the change in the confinement and interaction of gas molecules with the COF's pores as these interactions are dominant at low pressures as in VSA process. The change in APSs of COFs taken from different databases was mainly due to working capacities rather than selectivities as shown in Fig. S5.† The difference in structural properties between the CURATED and CoRE version of a COF significantly affected the CO2 uptakes (given in Fig. S6†), leading to considerably different working capacities. When we included the contribution of electrostatic interactions between COFs–guests in case (ii), larger changes were observed in APSs. The largest deviation in APS was observed in case (iii) due to the combined effect of structural discrepancy and different charges of CoRE COFs and CURATED COFs. In Fig. 3(a), the effect of different partial charges on APS values was clearly isolated for the COFs with ASACURATED/ASACoRE value of ∼1 (shown with standard symbol size). For one of these COFs, APS value decreased to half when the structure was taken from the CURATED database, showing the impact of using high accuracy charges on the performance predictions of COFs.
The top COFs selected for PSA process were found to have similar structures in CoRE and CURATED COF databases as shown by the size of the data points in Fig. 3(b). As a result, their predicted APSs were found to be strongly correlated for case (i) and (ii) leading to R2 values of 0.85 and 0.69 (excluding the COF with the highest deviation), respectively. The stronger correlation at PSA compared to VSA condition can be due to the COF–guest interactions which are less significant at higher pressure of PSA. However, again higher deviations were observed in APSs for case (iii) (R2 value of 0.27, excluding the same COF) due to the effect of DDEC charges on selectivity as shown in Fig. S7† with red points. We also computed APSs of the top COFs identified for PSA process at VSA conditions and showed them with hollow symbols in Fig. 3(b). APSs of these COFs were found to be correlated leading to R2 values of 0.78, 0.49, and 0.26 for case (i), (ii), and (iii), respectively. R2 values of these COFs were not as low as the ones obtained for the top COFs of VSA process, indicating that structural properties have less effect on the performance of the top COFs of the PSA process. Finally, for TSA process, most of the top COFs had high deviations in their ASAs and APSs upon structural curation as shown in Fig. 3(c) similar to the top COFs of VSA process. The calculated R2 values were 0.43 for case (i), 0.08 for case (ii), and 0.10 for case (iii). Both working capacity and selectivity changed when the curated structures were used in molecular simulations but the change in working capacity was more significant as shown in Fig. S8.†
It is not directly possible to apply detailed geometry optimization to a large number of COF structures and assign high accuracy partial charges. However, this comparison between databases suggested that predicted APSs of the top COFs whose structures were taken from CoRE COF database might be different from those taken from CURATED COF database, especially at low pressures where COF–gas interactions are important and more dependent on the structural properties. Sharma et al.72 similarly showed that optimized slipped COF structures have significantly different CO2/N2 selectivities than their counterparts. Therefore, detailed geometry optimization of COFs before simulations needs to be considered especially for VSA and TSA processes. We so far discussed the change in APS with respect to change in ASA, but change of APS with respect to changes in ρ and ϕ was also examined as shown in Fig. S9 and S10.† Overall, except for PSA, higher working capacities and higher selectivities were predicted when the structures were taken from CoRE database. As a result of this, higher APSs were obtained for CoRE COFs compared to CURATED COFs. Assigning partial charges to large numbers of COFs using approximate charge methods is computationally very efficient, however we suggest performing molecular simulations using CURATED COFs with high accuracy charges to make a final assessment about the potential of a COF adsorbent for VSA and TSA processes. We finally note that we only compared a small number of COFs from CoRE and CURATED databases and there is still much work needed to fully understand the dependence of simulated separation performance of COFs on the structures taken from different databases.
3.3 Membrane-based separation performance of COFs
We also examined membrane-based flue gas separation performances of 295 COFs. Adsorption, diffusion, and membrane selectivities of COFs computed at two different feed pressures, 1 and 10 bar, are shown in Fig. 4(a) and (b), respectively. Adsorption selectivities of all COFs were calculated to be greater than unity since CO2 is more strongly adsorbed than N2 as discussed in the previous section. Strong adsorption of CO2 molecules resulted in their slow diffusion and diffusion selectivity mostly favored N2. Diffusion of N2 was significantly higher than that of CO2 for 63 COFs at 1 bar and for 45 COFs at 10 bar as shown with black points in Fig. 4(a and b), respectively. In 23 (19) of these COFs at 1 bar (10 bar) CO2 adsorption selectivities dominated high N2 diffusion selectivity, leading to membranes with CO2 selectivities >2. However, using these COFs as adsorbents can still be a better choice since they have higher adsorption selectivities than their membrane selectivities. In a single COF at 1 bar (Fig. 4(a)) and 4 COFs at 10 bar (Fig. 4(b)), CO2 molecules diffuse faster than N2 molecules, shown by blue dots, resulting in diffusion selectivities for CO2 higher than unity. In these COFs, CO2 was favored both by adsorption and diffusion. The best selectivities for COF membranes were achieved when adsorption strongly favored CO2 and diffusion slightly favored N2, as shown by red points in Fig. 4. Membrane selectivities of small number of materials, 49 COFs at 1 bar and 23 COFs at 10 bar, were calculated to be less than 1, indicating that these COF membranes are N2 selective due to their high N2 diffusion selectivity dominating their CO2 adsorption selectivity. As a result of these, membrane selectivities of COFs were calculated to be between 0.38–21 and 0.63–37 at 1 bar and 10 bar, respectively.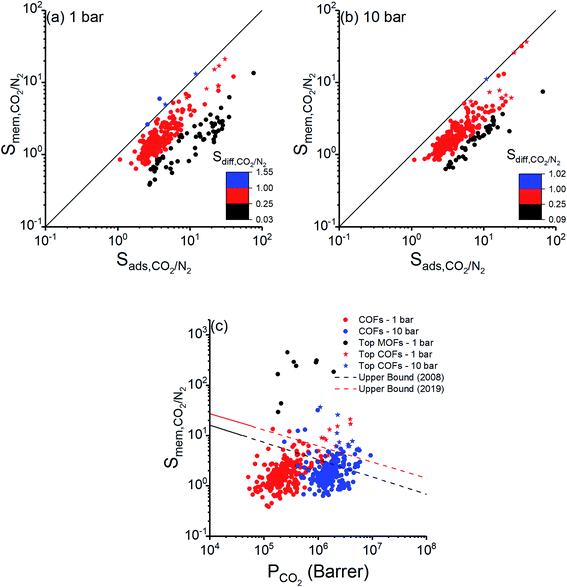 | ||
Fig. 4 Adsorption, diffusion, and membrane selectivities of COFs computed at (a) 1 bar, (b) 10 bar. Stars represent the top 10 COF membranes. (c) CO2 permeability and membrane selectivity of COFs for separation of CO2/N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15/85 mixture calculated at 1 and 10 bar presented together with upper bounds for polymeric membranes.73,74 Separation performances of the top MOF membranes are also shown for comparison of COFs with MOFs. 15/85 mixture calculated at 1 and 10 bar presented together with upper bounds for polymeric membranes.73,74 Separation performances of the top MOF membranes are also shown for comparison of COFs with MOFs. | ||
Fig. 4(c) represents CO2/N2 membrane selectivities of COFs as a function of CO2 permeabilities. CO2 selectivities and permeabilities of the best performing MOF membranes identified in a previous work of our group26 at 1 bar were also shown in Fig. 4(c). COF membranes offer similar permeabilities with the top MOF membranes, however selectivities of COF membranes were calculated to be lower, 0.38–21, than those of MOFs, 29–449. This can be explained by the large pore sizes and/or lack of metal sites in COFs as we discussed above. Robeson's upper bound,73 which is the upper limit for the separation performance of polymer membranes, and the new upper bound,74 which was recently updated considering CO2/N2 separation performances of ultrapermeable polymers of intrinsic microporosity, are also shown in Fig. 4(c). Due to their low selectivities, only a small number of COFs exceeded the upper bounds. On the other hand, we extended these upper bounds because COFs have very high CO2 permeabilities, ranging from 5.12 × 104 to 3.96 × 106 barrer at 1 bar and from 2.39 × 105 to 9.30 × 106 barrer at 10 bar. The most promising COF membranes offering CO2 permeabilities >106 barrer and the highest selectivities are shown by stars in Fig. 4(c) and detailed information about their calculated membrane metrics are given in Table 3.
| Top COFs | D self,CO2 (10−5 cm2 s−1) | D self,N2 (10−4 cm2 s−1) | P CO2 (106 barrer) | P N2 (105 barrer) | S ads,CO2/N2 | S diff,CO2/N2 | S mem,CO2/N2 |
|---|---|---|---|---|---|---|---|
| 1 bar | |||||||
| CC-TAPH-COF | 7.27 | 1.97 | 1.16 | 1.27 | 24.51 | 0.37 | 9.03 |
| COF-JLU3 | 8.88 | 2.49 | 1.27 | 1.43 | 24.91 | 0.36 | 8.87 |
| CTF-FUM | 28.50 | 4.18 | 2.12 | 3.48 | 9.02 | 0.68 | 6.15 |
| MPCOF | 55.20 | 5.08 | 1.03 | 2.11 | 4.55 | 1.09 | 4.94 |
| NPN-1 | 9.98 | 1.44 | 3.91 | 1.83 | 30.45 | 0.69 | 21.13 |
| NPN-2 | 12.00 | 1.78 | 3.96 | 2.32 | 25.26 | 0.67 | 16.98 |
| NPN-3 | 12.33 | 1.12 | 1.61 | 1.24 | 12.00 | 1.10 | 13.18 |
| PyTTA-BFBIm-iCOF | 8.01 | 1.66 | 1.39 | 1.69 | 16.96 | 0.48 | 8.18 |
| TEMPO-COF | 33.24 | 9.02 | 1.51 | 3.77 | 10.90 | 0.37 | 4.02 |
| TpMA | 17.16 | 2.46 | 2.27 | 1.50 | 21.77 | 0.70 | 15.19 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| 10 bar | |||||||
| CC-TAPH-COF | 6.58 | 1.40 | 4.28 | 5.52 | 16.58 | 0.47 | 7.78 |
| COF-JLU3 | 5.90 | 1.80 | 3.58 | 5.39 | 20.31 | 0.33 | 6.66 |
| CTF-1 | 26.44 | 3.20 | 5.46 | 9.90 | 6.64 | 0.83 | 5.49 |
| CTF-FUM | 6.33 | 6.20 | 2.22 | 2.00 | 10.91 | 1.02 | 11.14 |
| FLT-COF-1 staggered | 4.82 | 1.90 | 3.81 | 6.21 | 24.16 | 0.25 | 6.14 |
| NPN-1 | 1.15 | 1.23 | 1.09 | 0.30 | 39.39 | 0.93 | 36.56 |
| PyTTA-BFBIm-iCOF | 4.81 | 1.38 | 3.56 | 5.85 | 17.49 | 0.35 | 6.09 |
| TPE-COF-I | 4.34 | 7.11 | 2.46 | 3.35 | 12.03 | 0.61 | 7.34 |
| TpMA | 5.08 | 5.23 | 2.29 | 0.89 | 26.48 | 0.97 | 25.76 |
| TpPa-F4 | 5.13 | 1.55 | 2.22 | 3.71 | 18.17 | 0.33 | 6.02 |
3.4 Structure–performance analyses of COFs
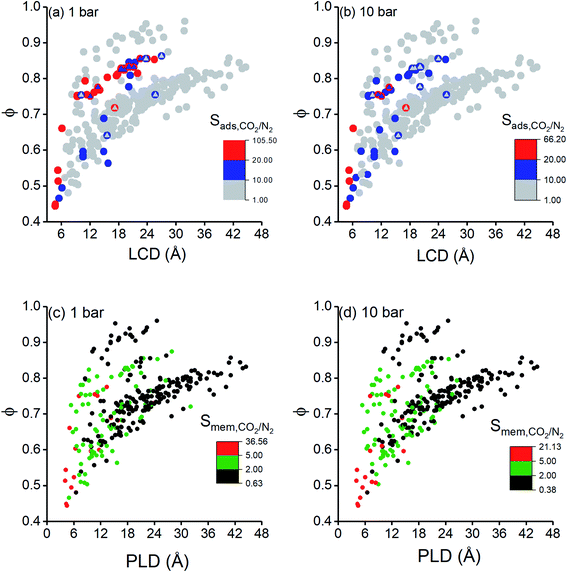
Finally, we investigated structure–performance relations of COFs to estimate the best combination of structural properties leading to a good CO2/N2 separation performance of a COF. Fig. 5(a and b) shows the relation between CO2/N2 selectivity of COF adsorbents calculated at 1 bar and 10 bar, porosity and LCD of 295 COFs. Selectivities of COFs increase as LCD and porosity decrease due to the better confinement of gas molecules inside the narrow pores.75 However, some COFs with LCD > 9 Å and ϕ > 0.6 did not follow the same trend, and their CO2/N2 selectivities were relatively high although they had large pores. To further investigate this, we performed GCMC simulations for these COFs by switching-off the coulombic interactions between COFs and adsorbate molecules. The triangles in Fig. 5(a and b) show selectivities of these COFs which were calculated by neglecting the coulombic interactions in molecular simulations. Selectivities significantly decreased when the electrostatic interactions were neglected and we changed the color of the symbols in Fig. 5(a and b) according to the coloring scale used for selectivity when all the interactions were considered in molecular simulations. The most remarkable difference was observed for the selectivity of ICOF-2, which decreased from 105 to 4, when the coulombic interactions between this COF and CO2 molecules were neglected. Electrostatic interactions between the host and adsorbates significantly contributed to the total energy of this COF, as discussed before, due to the existence of B and Li ions with large partial charges (1.28e− for B ions and 1.12e− for Li ions). Therefore, we concluded that COFs with large pores generally suffer from low adsorption selectivity but the ones having metal atoms with relatively large partial charges could offer high selectivities even if they are large-pored. Fig. 5(a) has a wider distribution of CO2/N2 selectivities and more outliers than Fig. 5(b) because the coulombic interactions between the adsorbent and adsorbates become more pronounced at low pressures. Similar to adsorption selectivity-LCD relation, we also studied membrane selectivity-PLD correlation. Fig. 5(c and d) shows the relation between CO2/N2 selectivity of COF membranes calculated at 1 bar and 10 bar, porosity and PLD of 295 COFs. Membrane selectivities of COFs decrease as their PLDs and porosities increase because both gases can easily diffuse through the large pores, limiting the separation capacity of membranes. Results in Fig. 5(a–d) showed that pore size has a greater importance than porosity on the CO2 selectivity of COFs since for a specific porosity a wide variety of selectivity values could be obtained according to the pore sizes of the COFs.We finally aim to elucidate the optimum structural properties of COFs to achieve high CO2/N2 separation performances and to compare these optimum structural properties with those of MOFs. Fig. 6 shows computed structural properties of all COFs that we studied in this work, the top 10 promising COF adsorbents identified for each cyclic adsorption process, VSA, PSA, and TSA, and the top 10 promising COF membranes. For both adsorption- and membrane-based flue gas separation, COFs with pore sizes (PLD and LCD) <10 Å offer the best performances. For adsorption-based separation, COFs with 2000 m2 g−1 < ASA <4500 m2 g−1 and 0.6 < ϕ <0.8 were identified to achieve the highest APS values whereas for membrane-based separation, COFs with ASA <2000 m2 g−1 and ϕ < 0.6 were identified to have the highest selectivities. Previously, it was shown that MOFs with pore sizes <7.5 Å, ASA <1000 m2 g−1, and 0.5 < ϕ < 0.75 can have high CO2/N2 adsorption and membrane selectivities.10,26 Promising COF adsorbents and COF membranes have slightly larger pore sizes, similar porosities, and higher surface areas compared to promising MOFs for CO2/N2 separation. We finally note that chemical properties of COFs, e.g. type of framework atoms, presence of metal atoms and functional groups in the frameworks, also effect CO2 adsorption, but we solely focused on the effects of structural properties, such as pore size and surface area which are easily calculated with computational methods, on the predicted separation performances of COFs in this work. These structure–performance relations will provide valuable information to experimentalists for the design and advancement of unique COF structures which realize highly selective CO2/N2 separations.
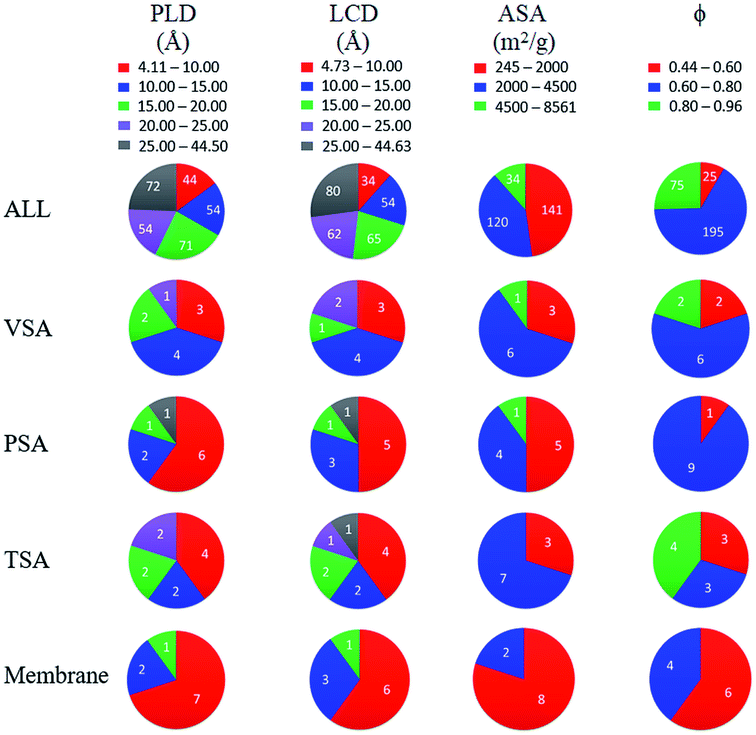 | ||
| Fig. 6 Distribution of calculated PLD, LCD, ASA and ϕ of all 295 COFs and the top 10 COFs identified for each separation process. Numbers in the charts show the number of COFs. | ||
4. Conclusion
In this work CO2/N2 mixture separation potentials of 295 COFs were examined both for adsorption- and membrane-based applications by combining GCMC and MD simulations. Results of molecular simulations were utilized for predicting adsorption selectivity, working capacity, R%, APS of COFs for three distinct cyclic adsorption processes, VSA, PSA, and TSA. Comparison of COFs with MOFs at VSA condition revealed that COFs have lower selectivities (1–105) than MOFs (2–6107) due to their larger pores and the lack of metal sites in their frameworks. On the other hand, COFs offer working capacities between 0.02–1.69 mol kg−1 while this range was 0.01–4.16 mol kg−1 for MOFs at VSA conditions. Results also showed that COFs can have higher selectivities than currently used adsorbents, such as zeolites and activated carbons, while having similar working capacities to them. Therefore, COFs have the potential to replace conventional adsorbents for CO2 separation from flue gas. For CO2/N2 separation using COFs as adsorbents, TSA process was suggested because more COFs were identified to have a combination of high R% (>85%) and high APS (>10 mol kg−1) compared to VSA and PSA conditions. Molecular simulations also showed that selectivity of COFs do not significantly change in the presence of humidity, making them potential adsorbents for wet flue gas separation. Curated, optimized COFs with high accuracy partial charges were computed to have lower APSs than CoRE COFs having approximate charges, especially if the structure was significantly affected from the geometry optimization. This showed the importance of using the correct COF structure before assessing its potential for CO2/N2 separation. Gas permeabilities of COF membranes (5.12 × 104 to 3.96 × 106 barrer) were computed to be similar to those of MOF membranes, however COFs suffered from low membrane selectivities (0.38–21). Due to their low selectivities, most COF membranes could not exceed the Robeson's upper bound.These results answered the question raised in the title of our work: COFs have the potential to compete with MOFs in adsorption-based flue gas separations but their potential as CO2 selective membranes is limited. However, several strategies such as functionalizing COFs by incorporating ionic liquids into COFs,76,77 assisting COFs with graphene oxides to fabricate ultrathin membranes,78 and using COFs as fillers in polymers to make mixed matrix membranes similar to MOFs26,79,80 can enhance membrane selectivities of COFs by tuning their pore sizes and/or chemical properties. Results of structure–performance analysis that we provided in this work can be used to advance the design and development of new COF adsorbents and membranes with desired structural properties. We note that COF structures that we studied in this work have been already experimentally synthesized and reported in the computation-ready COF database. Large-scale production of MOFs has already started so we expect to see scalable production and industrial applications of COFs especially in gas storage and separation fields in near future. Finally, high-throughput computational screening of COFs that we used in this work can be applied to unlock the potential of COFs to separate various gas mixtures in future studies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. K. acknowledges ERC-2017-Starting Grant. This study has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC-2017-Starting Grant, grant agreement No 756489-COSMOS).References
- T. Boden, R. Andres and G. Marland, Global, regional, and national fossil-fuel CO2 emissions (1751-2014)(v. 2017), Environmental System Science Data Infrastructure for a Virtual Ecosystem, 2017 Search PubMed.
- M. Songolzadeh, M. Soleimani, M. Takht Ravanchi and R. Songolzadeh, Sci. World J., 2014, 2014, 1–35 CrossRef PubMed.
- D. Bahamon and L. F. Vega, Chem. Eng. J., 2016, 284, 438–447 CrossRef CAS.
- N. Hedin, L. Andersson, L. Bergström and J. Yan, Appl. Energy, 2013, 104, 418–433 CrossRef CAS.
- R. Kumar, Ind. Eng. Chem. Res., 1994, 33, 1600–1605 CrossRef CAS.
- Y. S. Bae and R. Q. Snurr, Angew. Chem., Int. Ed., 2011, 50, 11586–11596 CrossRef CAS PubMed.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- J. Jiang, Mol. Simul., 2014, 40, 516–536 CrossRef CAS.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- C. Altintas, G. Avci, H. Daglar, A. Nemati Vesali Azar, S. Velioglu, I. Erucar and S. Keskin, ACS Appl. Mater. Interfaces, 2018, 10, 17257–17268 CrossRef CAS PubMed.
- S. Keskin, Nat. Energy, 2019, 5, 8–9 CrossRef.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974–988 CrossRef CAS PubMed.
- B. Liu and B. Smit, Langmuir, 2009, 25, 5918–5926 CrossRef CAS PubMed.
- B. Liu and B. Smit, J. Phys. Chem. C, 2010, 114, 8515–8522 CrossRef CAS.
- R. Krishna and J. M. J. S. van Baten, Sep. Purif. Technol., 2012, 87, 120–126 CrossRef CAS.
- Z. J. Zhang, Z. Z. Yao, S. C. Xiang and B. L. Chen, Energy Environ. Sci., 2014, 7, 2868–2899 RSC.
- Q. Wang, J. Bai, Z. Lu, Y. Pan and X. You, Chem. Commun., 2016, 52, 443–452 RSC.
- R. Ben-Mansour, M. A. Habib, O. E. Bamidele, M. Basha, N. A. A. Qasem, A. Peedikakkal, T. Laoui and M. Ali, Appl. Energy, 2016, 161, 225–255 CrossRef CAS.
- E. Haldoupis, S. Nair and D. S. Sholl, J. Am. Chem. Soc., 2012, 134, 4313–4323 CrossRef CAS PubMed.
- D. Wu, Q. Yang, C. Zhong, D. Liu, H. Huang, W. Zhang and G. Maurin, Langmuir, 2012, 28, 12094–12099 CrossRef CAS PubMed.
- C. E. Wilmer, O. K. Farha, Y. S. Bae, J. T. Hupp and R. Q. Snurr, Energy Environ. Sci., 2012, 5, 9849–9856 RSC.
- Z. W. Qiao, K. Zhang and J. W. Jiang, J. Mater. Chem. A, 2016, 4, 2105–2114 RSC.
- Y. G. Chung, J. Camp, M. Haranczyk, B. J. Sikora, W. Bury, V. Krungleviciute, T. Yildirim, O. K. Farha, D. S. Sholl and R. Q. Snurr, Chem. Mater., 2014, 26, 6185–6192 CrossRef CAS.
- R. Krishna and J. M. van Baten, Phys. Chem. Chem. Phys., 2011, 13, 10593–10616 RSC.
- T. Watanabe and D. S. Sholl, Langmuir, 2012, 28, 14114–14128 CrossRef CAS PubMed.
- H. Daglar and S. Keskin, J. Phys. Chem. C, 2018, 122, 17347–17357 CrossRef CAS PubMed.
- J. Liu, J. Tian, P. K. Thallapally and B. P. McGrail, J. Phys. Chem. C, 2012, 116, 9575–9581 CrossRef CAS.
- A. C. Kizzie, A. G. Wong-Foy and A. J. Matzger, Langmuir, 2011, 27, 6368–6373 CrossRef CAS PubMed.
- S. J. Datta, C. Khumnoon, Z. H. Lee, W. K. Moon, S. Docao, T. H. Nguyen, I. C. Hwang, D. Moon, P. Oleynikov, O. Terasaki and K. B. Yoon, Science, 2015, 350, 302–306 CrossRef CAS PubMed.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 4228–4235 CrossRef CAS PubMed.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- P. J. Waller, F. Gandara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923–933 CrossRef CAS PubMed.
- U. Díaz and A. Corma, Coord. Chem. Rev., 2016, 311, 85–124 CrossRef.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortes, A. P. Cote, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268–272 CrossRef CAS PubMed.
- M. Tong, Q. Yang and C. Zhong, Microporous Mesoporous Mater., 2015, 210, 142–148 CrossRef CAS.
- M. M. Tong, Y. S. Lan, Q. Y. Yang and C. L. Zhong, Chem. Eng. Sci., 2017, 168, 456–464 CrossRef CAS.
- M. M. Tong, Y. S. Lan, Z. L. Qin and C. L. Zhong, J. Phys. Chem. C, 2018, 122, 13009–13016 CrossRef CAS.
- T. Yan, Y. Lan, M. Tong and C. Zhong, ACS Sustainable Chem. Eng., 2018, 7, 1220–1227 CrossRef.
- D. Ongari, A. V. Yakutovich, L. Talirz and B. Smit, ACS Cent. Sci., 2019, 5, 1663–1675 CrossRef CAS PubMed.
- K. S. Deeg, D. D. Borges, D. Ongari, N. Rampal, L. Talirz, A. V. Yakutovich, J. M. Huck and B. Smit, ACS Appl. Mater. Interfaces, 2020, 12, 21559–21568 CrossRef CAS PubMed.
- T. F. Willems, C. H. Rycroft, M. Kazi, J. C. Meza and M. Haranczyk, Microporous Mesoporous Mater., 2012, 149, 134–141 CrossRef CAS.
- S.-Q. Xu, R.-R. Liang, T.-G. Zhan, Q.-Y. Qi and X. Zhao, Chem. Commun., 2017, 53, 2431–2434 RSC.
- Z. Sumer and S. Keskin, Ind. Eng. Chem. Res., 2016, 55, 10404–10419 CrossRef CAS.
- D. Dubbeldam, S. Calero, D. E. Ellis and R. Q. Snurr, Mol. Simul., 2016, 42, 81–101 CrossRef CAS.
- R. Ben-Mansour and N. A. Qasem, Energy Convers. Manage., 2018, 156, 10–24 CrossRef CAS.
- C. E. Wilmer and R. Q. Snurr, Chem. Eng. J., 2011, 171, 775–781 CrossRef CAS.
- R. L. Akkermans, N. A. Spenley and S. H. Robertson, Mol. Simul., 2013, 39, 1153–1164 CrossRef CAS.
- P. P. Ewald, Ann. Phys., 1921, 369, 253–287 CrossRef.
- B. L. Eggimann, A. J. Sunnarborg, H. D. Stern, A. P. Bliss and J. I. Siepmann, Mol. Simul., 2014, 40, 101–105 CrossRef CAS.
- J. J. Potoff and J. I. Siepmann, AlChE J, 2001, 47, 1676–1682 CrossRef CAS.
- C. Murthy, K. Singer, M. Klein and I. McDonald, Mol. Phys., 1980, 41, 1387–1399 CrossRef CAS.
- S. L. Mayo, B. D. Olafson and W. A. Goddard, J. Phys. Chem., 1990, 94, 8897–8909 CrossRef CAS.
- C. Vega, J. Abascal and I. Nezbeda, J. Chem. Phys., 2006, 125, 34503–34513 CrossRef CAS PubMed.
- J. L. Abascal and C. Vega, J. Chem. Phys., 2005, 123, 234505–234517 CrossRef CAS PubMed.
- B. Widom, J. Chem. Phys., 1963, 39, 2808–2812 CrossRef CAS.
- D. Frenkel and B. Smit, Understanding molecular simulation: From algorithms to applications, Elsevier (formerly published by Academic Press), 2002 Search PubMed.
- A. Einstein, Investigations on the theory of the Brownian movement, Courier Corporation, 1956 Search PubMed.
- S. Keskin and D. Sholl, Ind. Eng. Chem. Res., 2009, 48, 914–922 CrossRef CAS.
- I. Erucar and S. Keskin, J. Membr. Sci., 2016, 514, 313–321 CrossRef CAS.
- R. Poloni, K. Lee, R. F. Berger, B. Smit and J. B. Neaton, J. Phys. Chem. Lett., 2014, 5, 861–865 CrossRef CAS PubMed.
- C. Graham, D. A. Imrie and R. E. Raab, Mol. Phys., 1998, 93, 49–56 CrossRef CAS.
- Y. Basdogan, K. B. Sezginel and S. Keskin, Ind. Eng. Chem. Res., 2015, 54, 8479–8491 CrossRef CAS.
- Q. Lu, Y. Ma, H. Li, X. Guan, Y. Yusran, M. Xue, Q. Fang, Y. Yan, S. Qiu and V. Valtchev, Angew. Chem., Int. Ed., 2018, 57, 6042–6048 CrossRef CAS PubMed.
- F. J. Uribe-Romo, C. J. Doonan, H. Furukawa, K. Oisaki and O. M. Yaghi, J. Am. Chem. Soc., 2011, 133, 11478–11481 CrossRef CAS PubMed.
- P. Z. Moghadam, D. Fairen-Jimenez and R. Q. Snurr, J. Mater. Chem. A, 2016, 4, 529–536 RSC.
- H. Zhang and R. Q. Snurr, J. Phys. Chem. C, 2017, 121, 24000–24010 CrossRef CAS.
- I. Erucar and S. Keskin, Ind. Eng. Chem. Res., 2020, 59, 3141–3152 CrossRef CAS PubMed.
- A. Sharma, A. Malani, N. V. Medhekar and R. Babarao, CrystEngComm, 2017, 19, 6950–6963 RSC.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- B. Comesaña-Gándara, J. Chen, C. G. Bezzu, M. Carta, I. Rose, M.-C. Ferrari, E. Esposito, A. Fuoco, J. C. Jansen and N. B. McKeown, Energy Environ. Sci., 2019, 12, 2733–2740 RSC.
- G. Avci, S. Velioglu and S. Keskin, ACS Appl. Mater. Interfaces, 2018, 10, 33693–33706 CrossRef CAS PubMed.
- M. Zeeshan, V. Nozari, M. B. Yagci, T. Isık, U. Unal, V. Ortalan, S. Keskin and A. Uzun, J. Am. Chem. Soc., 2018, 140, 10113–10116 CrossRef CAS PubMed.
- L.-G. Ding, B.-J. Yao, F. Li, S.-C. Shi, N. Huang, H.-B. Yin, Q. Guan and Y.-B. Dong, J. Mater. Chem. A, 2019, 7, 4689–4698 RSC.
- Y. Ying, D. Liu, J. Ma, M. Tong, W. Zhang, H. Huang, Q. Yang and C. Zhong, J. Mater. Chem. A, 2016, 4, 13444–13449 RSC.
- X. Guo, Z. Qiao, D. Liu and C. Zhong, J. Mater. Chem. A, 2019, 7, 24738–24759 RSC.
- Y. Cheng, L. Zhai, Y. Ying, Y. Wang, G. Liu, J. Dong, D. Z. L. Ng, S. A. Khan and D. Zhao, J. Mater. Chem. A, 2019, 7, 4549–4560 RSC.
- M. Xu, S. Chen, D.-K. Seo and S. Deng, Chem. Eng. J., 2019, 371, 693–705 CrossRef CAS.
- P. J. Harlick and F. H. Tezel, Microporous Mesoporous Mater., 2004, 76, 71–79 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 2004, 49, 1095–1101 CrossRef CAS.
- E. García-Pérez, J. Parra, C. Ania, A. García-Sánchez, J. Van Baten, R. Krishna, D. Dubbeldam and S. Calero, Adsorption, 2007, 13, 469–476 CrossRef.
- X. Xu, X. Zhao, L. Sun and X. Liu, J. Nat. Gas Chem., 2008, 17, 391–396 CrossRef CAS.
- X. Dong, J. Zhang, L. Gang, P. Xiao, P. Webley and Y.-c. Zhai, J. Fuel Chem. Technol., 2011, 39, 169–174 CrossRef.
- A. L. Myers and J. M. Prausnitz, AlChE J, 1965, 11, 121–127 CrossRef CAS.
- D. Beaudoin, T. Maris and J. D. Wuest, Nat. Chem., 2013, 5, 830–834 CrossRef CAS PubMed.
- V. S. P. K. Neti, X. Wu, M. Hosseini, R. A. Bernal, S. Deng and L. Echegoyen, CrystEngComm, 2013, 15, 7157–7160 RSC.
Footnote |
† Electronic supplementary information (ESI) available: Comparison of CO2 uptakes obtained from GCMC simulations with experimental results; distribution of atoms for screened COFs; comparison of R% and Ndes values for TSA and VSA conditions; comparison of CO2 uptakes of CURATED and CoRE COFs obtained from GCMC simulations for CO2/N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15/85 mixture at VSA, PSA, and TSA conditions; comparison of calculated Sads and ΔN values of CURATED and CoRE COFs for VSA, PSA, and TSA conditions according to their ASA ratios; comparison of calculated APSs of CURATED and CoRE COFs according to their density and porosity ratios. See DOI: 10.1039/d0ta04574h 15/85 mixture at VSA, PSA, and TSA conditions; comparison of calculated Sads and ΔN values of CURATED and CoRE COFs for VSA, PSA, and TSA conditions according to their ASA ratios; comparison of calculated APSs of CURATED and CoRE COFs according to their density and porosity ratios. See DOI: 10.1039/d0ta04574h |
| This journal is © The Royal Society of Chemistry 2020 |