Creating compressive stress at the NiOOH/NiO interface for water oxidation†
Abstract
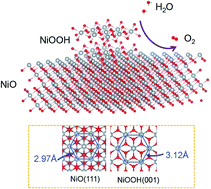
Enhancing the oxygen evolution reaction (OER) performance of NiO materials will greatly expand their applications as low-cost, bifunctional electrocatalysts for water splitting reactions. Introducing stress into the surface layer of catalysts represents an effective method to enhance their catalytic reactivity. Herein, we create compressive stress at the NiOOH/NiO interface using the battery conversion chemistry and in situ Ni to NiOOH transformation. As a result, the OER performance is enhanced by 20 fold compared with that of pure NiO. However, due to the corrosive environment that the electrocatalyst experiences under OER conditions, the stress is released after several CV cycles. The present study demonstrates the importance of interfacial stress that can be produced from in situ surface phase transformation of the electrocatalyst, as well as highlights the challenge of maintaining the mechanical stress under OER conditions during long-term applications.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...
