A function-switchable metal-free photocatalyst for the efficient and selective production of hydrogen and hydrogen peroxide†
Abstract
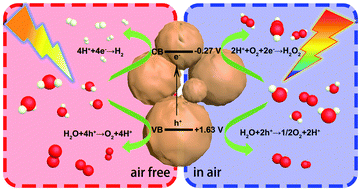
Photocatalytic water splitting to produce hydrogen and hydrogen peroxide is the main goal of photocatalysis. However, the reactions of both are water based and thus, it is extremely challenging to conduct both processes on one catalyst. Herein, a function-switchable metal-free catalyst was designed and synthesized through the polycondensation of procyanidin and 4-methoxybenzaldehyde. Impressively, the catalyst developed here not only split water to produce hydrogen via a four-electron reaction process in the absence of air, but also generated hydrogen peroxide by preferentially reducing oxygen via a two-electron pathway in air. The production rates of hydrogen and hydrogen peroxide reached 252.02 and 1385.42 μmol h−1 g−1, respectively. Through in situ transient photovoltage tests and kinetic analysis, a clear and fundamental understanding of the highly efficient and switchable functions was acquired for this photocatalyst. This study highlights the unique behavior and provides an insight into the design of function-switchable catalysts and the regulation of catalytic reactions.


 Please wait while we load your content...
Please wait while we load your content...