Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface functionalization of ZnO:Ag columnar thin films with AgAu and AgPt bimetallic alloy nanoparticles as an efficient pathway for highly sensitive gas discrimination and early hazard detection in batteries†
Alexander
Vahl
a,
Oleg
Lupan
 bc,
David
Santos-Carballal
bc,
David
Santos-Carballal
 *de,
Vasile
Postica
c,
Sandra
Hansen
*de,
Vasile
Postica
c,
Sandra
Hansen
 b,
Heather
Cavers
b,
Niklas
Wolff
f,
Maik-Ivo
Terasa
b,
Mathias
Hoppe
b,
Abdelaziz
Cadi-Essadek
b,
Heather
Cavers
b,
Niklas
Wolff
f,
Maik-Ivo
Terasa
b,
Mathias
Hoppe
b,
Abdelaziz
Cadi-Essadek
 e,
Torben
Dankwort
f,
Lorenz
Kienle
f,
Nora H.
de Leeuw
*dg,
Rainer
Adelung
b and
Franz
Faupel
e,
Torben
Dankwort
f,
Lorenz
Kienle
f,
Nora H.
de Leeuw
*dg,
Rainer
Adelung
b and
Franz
Faupel
 a
a
aInstitute for Materials Science – Chair for Multicomponent Materials, Faculty of Engineering, Christian-Albrechts-University of Kiel, Kaiserstraße 2, D-24143 Kiel, Germany. E-mail: ff@tf.uni-kiel.de
bInstitute for Materials Science – Functional Nanomaterials, Faculty of Engineering, Christian-Albrechts-University of Kiel, Kaiserstraße 2, D-24143 Kiel, Germany. E-mail: ra@tf.uni-kiel.de; ollu@tf.uni-kiel.de
cCenter for Nanotechnology and Nanosensors, Department of Microelectronics and Biomedical Engineering, Technical University of Moldova, 168 Stefan cel Mare Av., MD-2004 Chisinau, Republic of Moldova. E-mail: oleg.lupan@mib.utm.md
dSchool of Chemistry, University of Leeds, Leeds LS2 9JT, UK. E-mail: D.Santos-Carballal@leeds.ac.uk; N.H.deLeeuw@leeds.ac.uk
eSchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff CF10 3AT, UK
fInstitute for Materials Science – Synthesis and Real Structure, Faculty of Engineering, Christian-Albrechts-University of Kiel, Kaiserstraße 2, D-24143 Kiel, Germany
gDepartment of Earth Sciences, Utrecht University, Princetonplein 8A, 3584 CB Utrecht, The Netherlands. E-mail: n.h.deleeuw@uu.nl
First published on 11th May 2020
Abstract
For a fast and reliable monitoring of hazardous environments, the discrimination and detection of volatile organic compounds (VOCs) in the low ppm range is critically important, which requires the development of new chemical sensors. We report herein, a novel approach to tailor the selectivity of nanocomposite thin film sensors by investigating systematically the effect of surface decoration of Ag-doped ZnO (ZnO:Ag) columnar thin films. We have used AgPt and AgAu noble bimetallic alloy nanoparticles (NPs) to decorate the surfaces of ZnO:Ag and we have measured their resulting gas sensing properties towards VOC vapors and hydrogen gas. The gas response of the nanocomposite containing AgAu NPs to 100 ppm of ethanol, acetone, n-butanol, 2-propanol and methanol vapors was increased on average by a factor of 4 compared to the pristine ZnO:Ag columnar films. However, decoration with AgPt NPs led to a considerable reduction of the gas response to all VOC vapors and an increase of the response to H2 gas by roughly one order of magnitude, indicating a possible route to tailor the selectivity by surface decoration. As such, the reported NP-decorated ZnO:Ag thin film sensors should be suitable for the detection of H2 in Li-ion batteries, which is an early indication of the thermal runaway that leads to complete battery failure and possible explosion. To understand the impact of NP surface decoration on the gas sensing properties of ZnO:Ag thin films, we have employed density functional theory calculations with on-site Coulomb corrections and long-range dispersion interactions (DFT+U–D3-(BJ)) to investigate the adsorption of various VOC molecules and hydrogen onto the Ag-doped and NP-decorated (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface of zinc oxide ZnO. The calculated surface free energies indicate that Ag5Au5/ZnO(10
0) surface of zinc oxide ZnO. The calculated surface free energies indicate that Ag5Au5/ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag is the most favourable system for the detection of VOCs, which based on its work function is also the most reactive towards these species. Our calculated adsorption energies show that Ag9Pt/ZnO(10
0):Ag is the most favourable system for the detection of VOCs, which based on its work function is also the most reactive towards these species. Our calculated adsorption energies show that Ag9Pt/ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag has the largest preference for H2 gas and the lowest preference for the organic adsorbates, which is in line with the high selectivity of AgPt/ZnO:Ag sensors towards the hydrogen molecule observed in our experiments.
0):Ag has the largest preference for H2 gas and the lowest preference for the organic adsorbates, which is in line with the high selectivity of AgPt/ZnO:Ag sensors towards the hydrogen molecule observed in our experiments.
Introduction
Nanocomposites that consist of noble metal nanoparticles (NPs) and micro- and nanostructures of semiconducting oxides, such as ZnO, SnO2 and CuO, have attracted significant research interest for the their potentially sensitive detection of gases and VOC vapors,1–8 owing to their excellent electronic, catalytic and optical properties.8,9 Surface decoration by noble metal NPs has shown to affect many sensor characteristics including: (i) the sensitivity, enabling the detection of even trace amounts of gases; (ii) the selectivity towards certain gaseous species; (iii) the response and recovery times, which have been substantially reduced; and (iv) the acceptable operating temperatures, which have been shifted to values close to ambient conditions.1,10–12 Recently, highly sensitive and selective hydrogen gas nanosensors have been described that are based on individual ZnO nanowires decorated by Pd NPs.1,10 Guo et al. reported a remarkable enhancement of the gas sensing properties of ZnO nanorods by surface decoration with Au NPs, leading to fast response and recovery times, good selectivity and stable repeatability.11,13 Majhi et al. have successfully prepared Au@ZnO core–shell NPs by a facile low-temperature solution route for highly selective H2 gas detection, which was attributed to the chemical as well as catalytic effect of Au NPs.12 Khan et al. have successfully sensitized NiO–ZnO heterostructures with Ag2S by a cost-effective homogeneous precipitation method.14However, recent results have demonstrated that bimetallic NPs have unique physico-chemical properties, superior to their monometallic counterparts, owing to the combination of their unique and the induced synergistic effects.9,14–18 Yong et al. have used density functional theory calculations to investigate the adsorption of CO, HCN and NO on Ag7Au6 clusters for potential application as gas sensors and they found that adsorbates chemisorb on the surface of clusters with exothermic adsorption energies and finite charge transfer.16 Choi et al. decorated SnO2 nanowire networks with bimetallic PtPd NPs which led to fast response and recovery times during the detection of NO2.19 Fan et al. prepared ZnO loaded with PtAu bimetallic NPs, which showed high sensitivity to hydrogen down to the ppm-level, even at room temperature,20 whereas Hassan et al. decorated ZnO nanorods with PtPd bimetallic core–shell NPs for accelerated hydrogen gas detection.21
Thus, it is crucially important to develop a technological approach which can control alloyed NP properties15 and to investigate the gas sensing performance of decorated nanocomposites comprising micro- and nanostructured semiconducting oxides and bimetallic NPs. The Ag-based alloys, such as the bimetallic AgPt and AgAu NPs, are of particular interest due to the synergistic effect between the noble metal components, which makes them appealing for surface-enhanced Raman spectroscopy (SERS) as well as for applications in photocatalysis and sensing.9,22,23 Ag-based NPs have several advantages compared to other noble metals, e.g., harboring more active sites on their surface, which facilitates a more efficient electron transfer during the gas sensing applications.22 Ag-containing NPs are also more cost-effective in their preparation than their monometallic counterparts.22 The noble bimetallic AgAu NPs display a distinctive optical plasmon absorbance in the visible range.24 Their monometallic components have very similar lattice constants (4.09 Å for Ag and 4.08 Å for Au), allowing the preparation of different core–shell structures as well as alloys down to an average diameter of 4–5 nm,24 which are in high demand for device applications.
Despite their significant potential, the effect of AgAu and AgPt NPs decorating the surface of micro- and nanostructures of semiconducting oxides, especially columnar films, has not received much attention. Therefore, in the present study, nanocomposites were prepared by the decoration of Ag-doped ZnO columnar thin films (ZnO:Ag) with AgAu and AgPt NPs. Their gas sensing properties with regard to different VOCs and hydrogen gas were studied in detail. The sensors decorated with AgAu NPs-showed a significant increase in sensitivity towards VOCs compared to the pristine ZnO:Ag nanostructured films, whereas the sensors decorated using AgPt-displayed a change of selectivity from VOCs to H2 gas.
Furthermore, we can place the concept of noble metal alloy NP nanocomposite sensors in the context of early hazard detection in Li ion batteries (LIB). In this particular application scenario, the fast and reliable detection of H2 gas is especially important for the operation of battery modules that contain a large number of cells in parallel. The electrolytes are composed of a lithium salt and aprotic organic solvents, typically organic carbonates or ether solvents, which can produce H2 gas as a decomposition product.25–27 In this case, single battery cells inside a larger module experience degradation and a rise of their internal temperature to a critical point, which is likely to have a catastrophic effect on the performance of adjacent cells. In addition, in events like the sudden penetration or puncturing of the battery, the commonly used organic carbonate electrolytes decompose and produce large quantities of H2 gas, alongside a temperature increase of up to 200–250 °C. Thus, there is urgent demand for an immediate detection system with the potential for direct integration into the individual cells to avoid failure of the complete battery. In this study, the high sensitivity and fast response times of AgPt NP nanocomposite sensors is related to a potential application in this scenario.
Finally, the corresponding gas sensing mechanisms are proposed and supported by an extensive theoretical investigation. To uncover the origin of the unique gas sensing properties of the bimetallic alloy NP-decorated ZnO:Ag thin films, first principles simulations were applied to study the sensing properties towards VOCs of the Ag-doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface decorated with Ag5Au5 and Ag9Pt clusters, where the metal ratios were chosen for the simulations to reflect the similar composition of the experimentally obtained AgPt and AgAu NPs. The adsorption energies, electronic and structural implications have been investigated for different scenarios of substitutional doping and adsorption of the alloy metal clusters onto the surface. Furthermore, the binding of ethanol, acetone, n-butanol, 2-propanol, methanol and molecular hydrogen on the ZnO:Ag as well as the Ag9Pt/ZnO:Ag and Ag5Au5/ZnO:Ag modified surfaces are discussed to account for changes in selectivity and sensitivity. Whereas the sensitivity towards the VOC molecules was increased by the surfaces decorated with Ag5Au5 NPs, the Pt-based alloy NPs changed the nanocomposite's selectivity to H2 gas, in correspondence with the experiment.
0) surface decorated with Ag5Au5 and Ag9Pt clusters, where the metal ratios were chosen for the simulations to reflect the similar composition of the experimentally obtained AgPt and AgAu NPs. The adsorption energies, electronic and structural implications have been investigated for different scenarios of substitutional doping and adsorption of the alloy metal clusters onto the surface. Furthermore, the binding of ethanol, acetone, n-butanol, 2-propanol, methanol and molecular hydrogen on the ZnO:Ag as well as the Ag9Pt/ZnO:Ag and Ag5Au5/ZnO:Ag modified surfaces are discussed to account for changes in selectivity and sensitivity. Whereas the sensitivity towards the VOC molecules was increased by the surfaces decorated with Ag5Au5 NPs, the Pt-based alloy NPs changed the nanocomposite's selectivity to H2 gas, in correspondence with the experiment.
Experimental
Sample synthesis
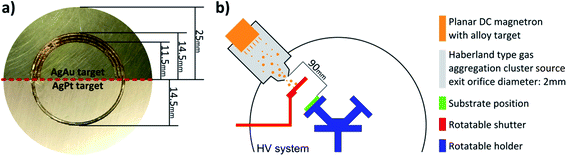
Ag-doped ZnO nanostructured columnar thin films (ZnO:Ag) with a nominal thickness of ∼1.5 μm were deposited on glass substrates by a simple and inexpensive synthesis method from chemical solutions (SCS). Details of the SCS method used to create the doped ZnO columnar films were presented in previous works.28–31 The Ag doping content in the prepared samples was adjusted to 0.95 wt%, which was confirmed in an earlier investigation by energy-dispersive X-ray (EDX) spectroscopy and X-ray photoelectron spectroscopy (XPS).32 All samples were thermally annealed at 650 °C for 2 h in air after deposition.The noble bimetallic alloy NPs were deposited onto the ZnO:Ag columnar thin films and Si wafer pieces (for reference measurements, Si-Mat, 10 × 10 mm2) using a custom-built high vacuum deposition system with an in-house Haberland type Gas Aggregation Source (GAS).33–35 For the generation of the AgAu and AgPt bimetallic alloy NPs, customized silver targets (Kurt J. Lesker, 99.99%, 50.8 mm diameter) were used, with a drilled trench and a fitted Au wire (Alfa Aesar, 1.0 mm diameter, 99.95%) or Pt wire (Alfa Aesar, 1.0 mm diameter, 99.95%), respectively. The deposition of alloy NPs by applying the target geometry methodology has been characterized in detail in an earlier work.36 A photograph depicting both targets in the geometry used is given in Fig. 1a, while the whole PVD setup is sketched in Fig. 1b. The respective target is mounted onto a DC planar magnetron source (Thin Film Consulting, ION'X-2UHV). Prior to deposition, the HV deposition system was evacuated to at least 10−4 Pa using a turbo molecular pump (Pfeiffer Vacuum, TMU 262) and a dry scroll pump (Agilent Technologies, SH-110). For sputtering, an Ar flow of 48 sccm, (purity 99.999%) was supplied at the gas inlet near the target (gas regulating valve: Pfeiffer, EVR116 with a hot ion cathode IMR 285 attached), resulting in a pressure of typically 136 Pa inside the GAS. For sputtering, a DC power of 40 W was supplied by the power source (Advanced Energy, MDX 500). Prior to every deposition, the target was cleaned and the NP growth was conditioned with a closed shutter for the time (at least 30 s) required to reach stable deposition conditions.
Sample characterization
Transmission Electron Microscopy (TEM) was used to investigate the nanostructure of the NP-decorated ZnO:Ag thin films using a Tecnai F30 G2 microscope (field emission gun, 300 kV) equipped with a Si(Li) EDX detector (EDAX system). TEM samples were prepared by scratching the nano–micro-columnar film containing the bimetallic clusters with a sharp knife and by subsequently transferring the resulting powder to a carbon lacey Cu grid by touching the grid onto the powder.Analogous to previous works, for the electrical measurements, interdigitating Au electrodes were deposited by sputter deposition on top of the thin film.29,37 The gas sensing properties were measured as reported previously.28,38,39 Throughout this study, a typical concentration of 100 ppm of the respective gas species was applied to the gas sensors. To account for the low gas response of AgAu/ZnO:Ag and ZnO:Ag sensors towards hydrogen gas, the concentration in this test scenario was raised to 1000 ppm. The gas sensing properties were studied in a typical range of operating temperatures between 200 °C and 350 °C. The electrical measurements were performed using a Keithley 2400 SourceMeter, controlled by a LabView program (National Instruments).37
X-ray photoelectron spectroscopy (XPS, Omicron Nano-Technology GmbH, Al-anode, 240 W) was applied to investigate the chemical composition of the deposited NPs and nanocomposites. The C-1s line of adventitious carbon at roughly 285.0 eV was used for charge referencing of all recorded spectra. The software CasaXPS (version 2.3.16) was used to quantify the composition of the noble bimetallic AgAu and AgPt alloy NPs. Micro-Raman studies were performed at room temperature with a WITec alpha 300 RA system in backscattering configuration. The Nd-YAG laser power at the laser position was less than 4 mW.39
Calculation details
Density functional theory (DFT) calculations of the interaction of a number of VOCs and molecular hydrogen with the ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag, Ag5Au5/ZnO(10
0):Ag, Ag5Au5/ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag and Ag9Pt/ZnO(10
0):Ag and Ag9Pt/ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag decorated surfaces are also reported. All calculations were performed using the Vienna Ab initio Simulation Package (VASP).40–43 The valence electronic states were expanded via a plane-wave basis set with a kinetic energy cut-off of 400 eV. The projector augmented wave (PAW) method44,45 was used to represent the interactions between the core (Au, Pt: [Xe] 4f14; Ag: [Kr]; Zn: [Ar]; O, C: [He]) and the valence electrons. For the H atoms, the electron was treated as valence. The method of Grimme with Becke–Johnson damping [D3-(BJ)]46,47 was applied to improve the description of the long-range dispersion forces. The simulations were carried out using the Perdew, Burke, and Ernzerhof (PBE)48 exchange–correlation functional within the generalised gradient approximation (GGA). An effective Ueff value of 6.0 eV (ref. 39 and 49) was applied to the strongly correlated and localised Zn 3d electrons to enhance the description of their on-site Coulomb interactions.50,51 However, no Hubbard Hamiltonian was employed for either the transition metal dopant or clusters states, as they are already delocalised given their low concentration52 and metallic properties, respectively. The structures were optimised using the conjugate-gradients technique and were considered converged when the forces on all ions were less than 0.01 eV Å−1. All calculations were spin-polarised to describe properly the unpaired electrons produced upon Zn(II) substitution by Ag(I).
0):Ag decorated surfaces are also reported. All calculations were performed using the Vienna Ab initio Simulation Package (VASP).40–43 The valence electronic states were expanded via a plane-wave basis set with a kinetic energy cut-off of 400 eV. The projector augmented wave (PAW) method44,45 was used to represent the interactions between the core (Au, Pt: [Xe] 4f14; Ag: [Kr]; Zn: [Ar]; O, C: [He]) and the valence electrons. For the H atoms, the electron was treated as valence. The method of Grimme with Becke–Johnson damping [D3-(BJ)]46,47 was applied to improve the description of the long-range dispersion forces. The simulations were carried out using the Perdew, Burke, and Ernzerhof (PBE)48 exchange–correlation functional within the generalised gradient approximation (GGA). An effective Ueff value of 6.0 eV (ref. 39 and 49) was applied to the strongly correlated and localised Zn 3d electrons to enhance the description of their on-site Coulomb interactions.50,51 However, no Hubbard Hamiltonian was employed for either the transition metal dopant or clusters states, as they are already delocalised given their low concentration52 and metallic properties, respectively. The structures were optimised using the conjugate-gradients technique and were considered converged when the forces on all ions were less than 0.01 eV Å−1. All calculations were spin-polarised to describe properly the unpaired electrons produced upon Zn(II) substitution by Ag(I).
The calculations of the insulating surfaces and isolated molecules were carried out sampling only the Γ point in the reciprocal space. However, the metallic phases were modelled using a Γ-centred Monkhorst–Pack mesh53 with a maximum separation of 0.17 Å−1 between k-points. The partial occupancies for the surfaces and isolated molecules were determined using the Gaussian smearing method,54 with a width set at 0.05 eV. For the geometry optimisation of the metallic phases, the partial occupancies were simulated using the Methfessel–Paxton scheme order 1 with a smearing width of 0.10 eV. However, the tetrahedron method with Blöchl corrections was used for the static calculations of the metallic phases to simulate the magnetic and electronic properties and to obtain accurate total energies. These criteria allowed convergence of the total energy within 10−4 eV per atom.
Results and discussion
Morphological, structural and micro-Raman characterizations
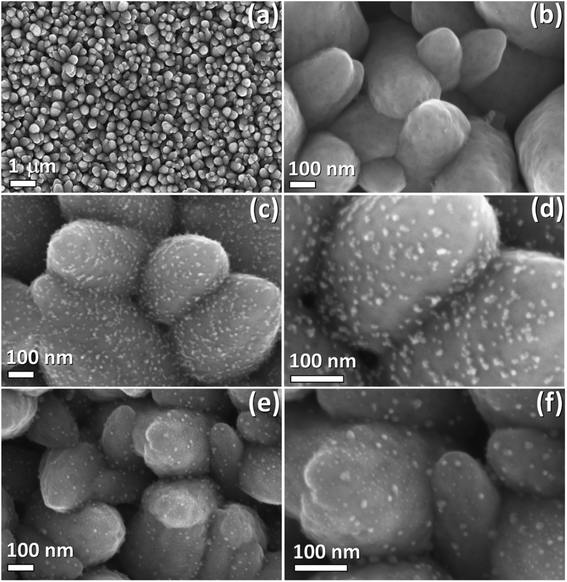
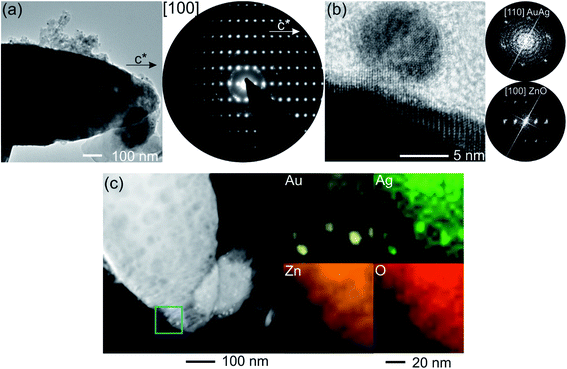
SEM micrographs of pristine ZnO:Ag columnar thin films, as well as of nanocomposites decorated with AgAu and AgPt bimetallic alloy NPs, are depicted in Fig. 2. The morphology of a typical pristine ZnO:Ag thin film after heat treatment at 650 °C for 2 h (Fig. 2a and b) consists of closely packed and partially interconnected grains. The diameter of these grains varies between 50 and 400 nm. SEM micrographs of ZnO:Ag columnar thin films, which were decorated with AgPt NPs, are displayed in Fig. 2c and d. The bimetallic AgPt NPs have a diameter ranging between 5 and 15 nm and are well distributed on the surface of the ZnO:Ag columnar thin films, showing no formation of percolating paths. However, due to the stochastic character of the deposition method, occasional agglomeration of small numbers of NPs is observed (see Fig. 2d). The SEM micrographs showing ZnO:Ag thin films decorated by AgAu NPs are presented in Fig. 2e and f. In this case, the deposited NPs exhibit typical NP diameters between 3 nm and 18 nm (see Fig. 2f). In both AgPt and AgAu alloy NPs, the mean diameter is considerably smaller than the grain diameter of the ZnO:Ag thin film. These observed diameters, as well as the sparse deposition with occasional agglomerations, agrees well with a typical NP deposition via GAS well below the percolation threshold.36Moreover, TEM experiments were conducted to investigate the nanostructure and verify the presence of bimetallic alloy NPs. For example, Fig. 3a depicts an overview image of an AgAu/ZnO:Ag nanocomposite. The electron diffraction (ED) pattern, recorded at the tip of the displayed AgAu/ZnO:Ag grain, provides information that the doped columnar grains grow as single crystals along the c-axis of the wurtzite-type structure. Furthermore, the ED pattern exhibits weaker diffraction intensities distributed on rings. The appearance of these rings is attributed to polycrystalline ZnO, which is partially adhered to the surface and alloy NPs. High resolution TEM micrographs (see Fig. 3b) show the presence of NPs with diameters in the range of 5–20 nm, which is in good agreement with the observations from the SEM micrographs of Fig. 2f. The imaged NPs seem to be loosely bound to the ZnO surface and do not show a systematic intergrowth structure. The Fourier transform of the matrix verifies the presence of a single crystalline ZnO (zone axis [100], [21−10] in hexagonal notation) phase. Due to the similar crystal structures of Ag and Au, the composition of the NPs could not be distinguished between the pure metals or alloy (zone axis [110]). To verify the presence of AgAu NPs (or AgPt NPs), the nanocomposite was investigated by EDX elemental mapping (see Fig. 3c or S2,† respectively). The EDX elemental map (Fig. 3c) clearly shows the location of the overlapped signals for the high Ag and Au, which indicates the presence of AgAu alloy NPs. A similar trend is observed in the EDX signals for Ag and Pt in the AgPt/ZnO:Ag nanocomposite (Fig. S2†). It is reported that Ag and Pt form a miscibility gap ranging roughly from 13 at% to 98 at%, with respect to Pt content.55,56 However, phase separation could not be identified within the measuring limits of the microscope used (Fig. S2†). Considering that Pt content is about 12 at% (see XPS studies below), it is reasonable to assume that a solid solution has formed.
Fig. S1† shows the Raman spectra of the AgAu- and AgPt-decorated ZnO:Ag columnar films. For all samples, the two dominant peaks were observed at ∼100 cm−1 and ∼438 cm−1, which are attributed to the E2(low) and E2(high) modes of the wurtzite structure of ZnO, respectively. Other peaks with lower intensities were observed at ∼337, ∼380, ∼410, ∼576 and ∼584 cm−1, which are attributed to E2(high)–E2(low), A1(TO), E1(TO), A1(LO) and E1(LO) modes, respectively.30,31
XPS studies
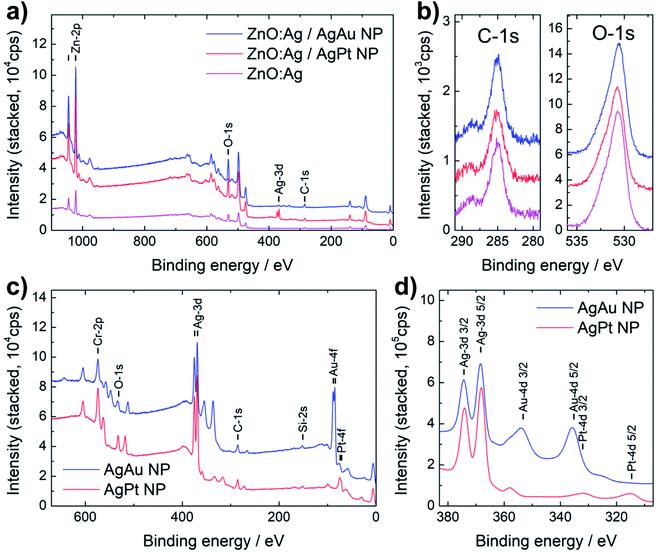
The ZnO:Ag nanocolumnar thin film (as a reference layer), as well as the nanocomposites decorated with the AgPt and AgAu alloy NPs, were investigated by XPS. Fig. 4a shows the overview spectra of the nanocomposites (AgAu: red line, AgPt: blue line) and the reference layer (magenta, bottom line). Based on the characteristic peaks of these spectra, the elemental composition is determined. The elements Zn, O, C and Au are detected in all spectra. The position of line C-1s in Fig. 4b, which was used for charge referencing, indicates atmospheric surface contamination from organic compounds, such as carbohydrates.32 The prominent signal of Zn and O is attributed to the ZnO base layer. The comparison of the O-1s high resolution spectra (Fig. 4b) indicates a similar peak position and shape for the investigated nanocomposites as well as for the pristine substrate. Ag is detected in all three samples, but the corresponding signal is rather weak, especially for the ZnO:Ag base layer, which is attributed to the low doping concentration of Ag. A comparison of high resolution spectra of the Ag-3d, Au-4d and Pt-4d lines can be found in Fig. S3.† The signal is stronger in the case of the AgAu NPs and AgPt NPs decorated nanocomposites, but due to the intentionally low surface coverage of NP, the signal is still weak for a precise quantification.For further investigation of the deposited NPs, the reference spectra of AgPt and AgAu alloy NPs (deposited on silicon wafer pieces) are compared in Fig. 4c and d. The overview spectra in Fig. 4c indicates the presence of Si, Cr, O, C, Ag and Au or Pt for the AgAu and AgPt NPs, respectively. To quantify the composition of the bimetallic alloy NPs, a detailed binding energy scan of the Ag-3d, Au-4d and Pt-4d lines from 382 eV to 313 eV is shown in Fig. 4d. Additional peaks with an offset to lower binding energies (by roughly 10 eV) occur in the spectra, due to the use of a non-monochromated X-ray source. In case of the AgAu NPs, the offset peak from Ag-3d5/2 overlaps with the Au-4d3/2 line. Accordingly, the Au-4f line was used for the quantification of Au, which was estimated at 52 at% in the AgAu NPs. For the AgPt NPs, the relevant Ag-3d and Pt-4d lines are well separated and correspond to a Pt content of roughly 12 at%. Considering the lower sputtering rate of Pt compared to Au (roughly 0.57 of Au) and the difference in target design (three Au wires for AgAu and one Pt wire for AgPt), the difference in concentration of Ag in the AgPt and AgAu NPs is expected.33–36 The estimated composition of AgPt and AgAu NPs is presented in Table S1 in the ESI.†
Gas sensing properties
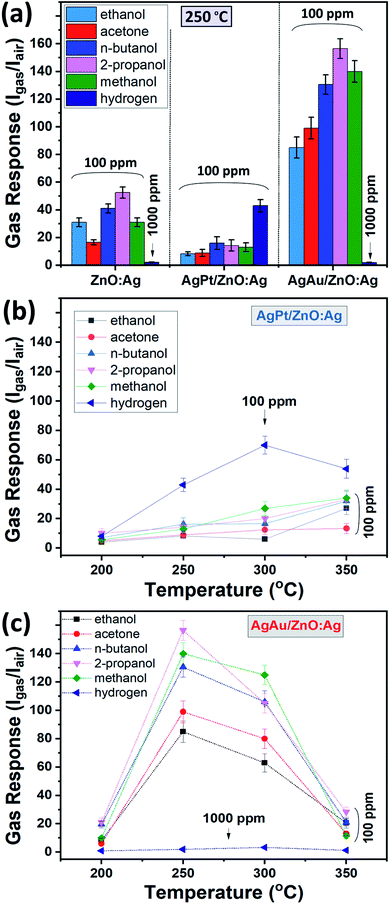
Fig. 5a shows the gas response of the pristine ZnO:Ag, AgPt/ZnO:Ag and AgAu/ZnO:Ag nanocomposites to hydrogen gas (H2) and different VOC vapors, including ethanol, acetone, n-butanol, 2-propanol and methanol at an operating temperature of 250 °C. In a typical gas sensing experiment, the thin film sensor was exposed to a concentration of 100 ppm of each VOC or H2. However, in the case of the ZnO:Ag and AgAu/ZnO:Ag columnar films, an H2 concentration of 1000 ppm was used to underline the low sensitivity of these sensors towards H2. A considerable increase of the response to VOC vapors was observed for the ZnO:Ag columnar films that were decorated with AgAu NPs. The gas response of the ZnO:Ag columnar films to 1000 ppm of hydrogen and 100 ppm of ethanol, acetone, n-butanol, 2-propanol and methanol is ∼2.1, ∼31, ∼16.5, ∼41, ∼52.5 and ∼31, respectively, while for AgAu/ZnO:Ag it is ∼2, ∼85, ∼99, ∼130.5, ∼156.5 and ∼140, respectively. Compared to the pristine ZnO:Ag thin film, the gas response of AgAu/ZnO:Ag to ethanol, acetone, n-butanol, 2-propanol and methanol was increased by factor of ∼2.8, ∼6, ∼3.2, ∼3 and ∼4.5, respectively. The gas response values are also considerably higher compared to those obtained for ZnO:Ag columnar films decorated with monometallic Ag NP-synthesized by the same method,32 proving the higher efficiency of bimetallic NP decoration. The low gas response to hydrogen gas is characteristic for all samples, with the exception of the AgPt/ZnO:Ag columnar films. Although the decoration with the AgPt alloy NPs leads to a decrease in response for all the VOCs tested, by a factor of 2, it enhances the sensitivity to 100 ppm of H2 gas by a factor 40. This is a large change compared to the almost no response to H2 gas in the case of the reference and the AgAu decorated sample sets, which indicates the possibility of tailoring the selectivity of ZnO:Ag thin film sensors by surface decoration with noble bimetallic alloy NPs of various composition. In addition to contemporary approaches, e.g. incorporating molecular sieves,57 the versatility of GAS to produce a variety of metal, metal alloy and metal oxide NPs opens up a highly interesting research field for further improvements in sensor selectivity.Fig. 5b and c show the dependence of the gas response, to H2 and different VOC vapors with respect to the operating temperature for the AgPt/ZnO:Ag and AgAu/ZnO:Ag nanocomposites, respectively. In both cases, the optimal operating temperature is 250–300 °C. Since no gas response higher than 1.5 was observed at operating temperatures below 200 °C, this temperature regime was not investigated further.
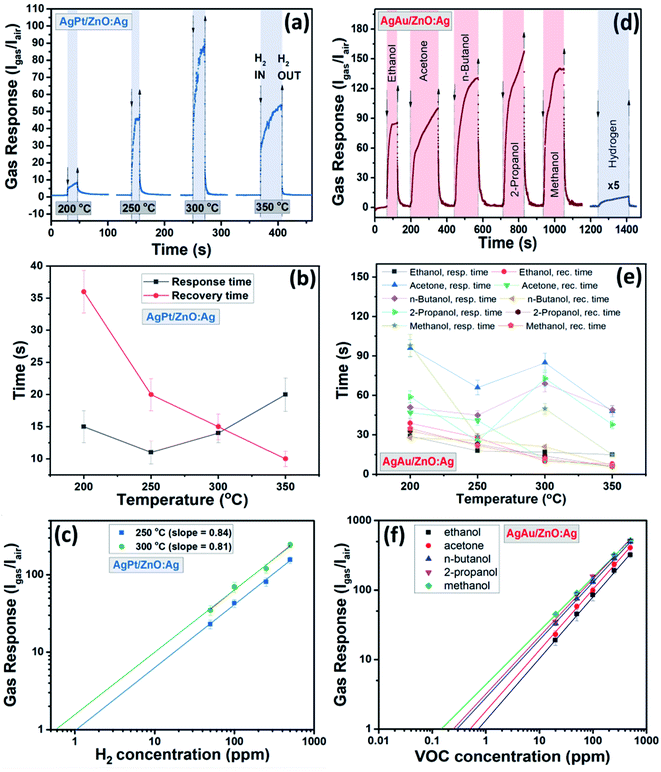
The dynamic response of the AgPt/ZnO:Ag columnar films to 100 ppm of H2 gas at different operating temperatures (200–350 °C) is presented in Fig. 6a. The calculated values for the associated response and recovery times are presented in Fig. 6b. The response times are confined to a small range (11–20 s), while the recovery times decrease from 36 s to 10 s when the operating temperature rises from 200 to 350 °C.
The dependence of the H2 gas response (SH2) to its concentration at 250 and 300 °C is presented in Fig. 6c. A power law relationship is observed (SH2 ∝ pH2β), where β, which is the slope of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SH2vs. log
SH2vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pH2,39 has a value of β ≈ 0.85 at both 250 and 300 °C. The detection limits calculated for the H2 gas (criterion for gas detection: Igas/Iair > 1.2 (ref. 58)) were ∼1 and ∼0.6 ppm at 250 and 300 °C, respectively.
pH2,39 has a value of β ≈ 0.85 at both 250 and 300 °C. The detection limits calculated for the H2 gas (criterion for gas detection: Igas/Iair > 1.2 (ref. 58)) were ∼1 and ∼0.6 ppm at 250 and 300 °C, respectively.
The dynamic responses of the AgAu/ZnO:Ag columnar films to 100 ppm of VOC vapors at their optimal operating temperatures of 250 °C are presented in Fig. 6d. All samples show fast signal recovery to the initial electrical baseline after evacuation of the vapors from the test chamber, which is essential for the reliability of sensors and their practical applications. The calculated response and recovery times versus the operating temperature are presented in Table 1 and Fig. 6e. The recovery times for all samples are comparable to one of the best results reported for functionalized/decorated ZnO samples (see Table 2).
| Temperature, °C | ||||||
|---|---|---|---|---|---|---|
| 200 | 250 | 300 | 350 | |||
| ZnO:Ag | Ethanol | Resp. time (s) | 62 | 49 | 22 | 18 |
| Rec. time (s) | 16 | 9 | 3 | 2 | ||
| Acetone | Resp. time (s) | 114 | 65 | 41 | 18 | |
| Rec. time (s) | 47 | 11 | 3 | 2 | ||
| n-Butanol | Resp. time (s) | 88 | 105 | 43 | 38 | |
| Rec. time (s) | 22 | 10 | 4 | 3 | ||
| 2-Propanol | Resp. time (s) | 57 | 65 | 38 | 24 | |
| Rec. time (s) | 16 | 6 | 4 | 3 | ||
| Methanol | Resp. time (s) | 80 | 51 | 17 | 40 | |
| Rec. time (s) | 28 | 10 | 4 | 3 | ||
| AgPt/ZnO:Ag | Hydrogen | Resp. time (s) | 15 | 11 | 14 | 20 |
| Rec. time (s) | 36 | 20 | 15 | 10 | ||
| Acetone | Resp. time (s) | 78 | 21 | 29 | 20 | |
| Rec. time (s) | 36 | 11 | 5 | 3 | ||
| n-Butanol | Resp. time (s) | 61 | 6 | 28 | 8 | |
| Rec. time (s) | 29 | 7 | 5 | 3 | ||
| 2-Propanol | Resp. time (s) | 113 | 94 | 43 | 40 | |
| Rec. time (s) | 43 | 15 | 3 | 2 | ||
| Methanol | Resp. time (s) | 46 | 23 | 45 | 34 | |
| Rec. time (s) | 32 | 8 | 4 | 2 | ||
| AgAu/ZnO:Ag | Ethanol | Resp. time (s) | 29 | 18 | 17 | 15 |
| Rec. time (s) | 39 | 28 | 10 | 8 | ||
| Acetone | Resp. time (s) | 96 | 66 | 85 | 48 | |
| Rec. time (s) | 47 | 41 | 11 | 6 | ||
| n-Butanol | Resp. time (s) | 51 | 45 | 69 | 49 | |
| Rec. time (s) | 28 | 26 | 21 | 7 | ||
| 2-Propanol | Resp. time (s) | 59 | 25 | 73 | 38 | |
| Rec. time (s) | 33 | 23 | 14 | 6 | ||
| Methanol | Resp. time (s) | 98 | 29 | 50 | 15 | |
| Rec. time (s) | 35 | 22 | 12 | 6 | ||
| Sensing material | VOCs conc. (ppm) | Gas response (Igas/Iair) or (Rair/Rgas) | Operating temperature (°C) | Response time (s) | Recovery time (s) |
|---|---|---|---|---|---|
| Ag–ZnO films84 | Ethanol 2000 | ∼2 | 225 | 5 | — |
| Ag/ZnO nanorods85 | Ethanol 100 | 36.52 | 360 | 50 | 28 |
| ZnO–Ag hybrids86 | Ethanol 100 | 101.8 | 370 | ∼15 | ∼20 |
| Ag–ZnO nanorods87 | Ethanol 50 | 34.8 | 280 | — | — |
| Acetone 50 | 25 | ||||
| Methanol 50 | 14.5 | ||||
| Ag-loaded ZnO88 | Ethanol 100 | ∼75 | 240 | — | — |
| Acetone 100 | ∼30 | ||||
| Isopropanol 100 | ∼68 | ||||
| Methanol 100 | ∼55 | ||||
| Au-doped ZnO NWs89 | Ethanol 1000 | ∼37 | 240 | — | — |
| Au/ZnO NWs11 | Ethanol 100 | 33.6 | 380 | 3 | 1 |
| Au/ZnO nanorods90 | Ethanol 100 | 89.5 | 300 | 2 | 2 |
| Au NPs/ZnO91 | Ethanol 1000 | 32 | 300 | — | — |
| Au/ZnO nanoplates77 | Ethanol 100 | 21 | 400 | 2 | 2 |
| Acetone 100 | 32 | 4 | 4 | ||
| Au/ZnO nanoplates92 | Ethanol 100 | 20 | 300 | 13 | — |
| Acetone 100 | 16 | — | — | ||
| Methanol 100 | 7 | — | — | ||
| Au/ZnO yolk–shell nanospheres70 | Acetone 100 | 37 | 300 | 2 | 38 |
| Methanol 100 | ∼22 | — | — | ||
| Au/ZnO71 | Ethanol 50 | 8.9 | 300 | 10 | — |
| Methanol 50 | ∼7 | ||||
| Au@ZnO core–shell nanoparticles12 | Ethanol 100 | ∼63 | 300 | 75 | 600 |
| Au/T-TiZnO93 | Ethanol 50 | ∼22 | 320 | — | — |
| Au/flower-like ZnO69 | Ethanol 100 | 45.56 | 270 | — | — |
| Acetone 100 | 74.41 | 5 | 3 | ||
| Pt/ZnO NWs94 | Ethanol 50 | 32.6 | 265 | — | — |
| PtAu/ZnO nanorods20 | H2 250 | ∼160 | 130 | — | — |
| PtPd/ZnO nanorods21 | H2 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
∼3 | 100 | 5 | 76 |
| AgPt/ZnO:Ag columnar films (this work) | H 2 100 | 43 | 250 | 11 | 20 |
| 70 | 300 | 14 | 15 | ||
| Ethanol 100 | 8.3 | 250 | 7 | 12 | |
| Acetone 100 | 8.9 | 250 | 21 | 11 | |
| 2-Propanol 100 | 14.3 | 250 | 6 | 7 | |
| n-Butanol 100 | 16.2 | 250 | 94 | 15 | |
| Methanol 100 | 13 | 250 | 23 | 8 | |
| AgAu/ZnO:Ag columnar films (this work) | Ethanol 100 | 85 | 250 | 18 | 28 |
| Acetone 100 | 99 | 66 | 41 | ||
| 2-Propanol 100 | 156.5 | 45 | 26 | ||
| n-Butanol 100 | 130.5 | 25 | 23 | ||
| Methanol 100 | 140 | 29 | 22 | ||
| Hydrogen 1000 | 2 | 64 | 21 |
Table 2 shows other results for high performance VOC vapors sensors based on ZnO micro- and nanostructures functionalized/decorated/modified with monometallic and noble bimetallic (Au, Ag, Pt) NPs. This table clearly demonstrates that our results are equivalent or superior to the performances reported for highly sensitive VOC sensors, although there are several reports of H2 response values much higher than for our AgPt/ZnO:Ag columnar films.1,10,59–61 However, the remarkable point of our materials is the possibility to change the selectivity from VOC vapors to H2 gas, which opens the opportunity for the fabrication of highly selective gas sensors. In addition, the platinum content in the bimetallic AgPt NPs, which in this work was determined to be roughly 12 at%, results in a more cost-effective approach to tailor the sensor selectivity compared to the use of the pure material.
The dependence of the gas response on the concentration of VOC vapors for the AgAu/ZnO:Ag columnar films is presented in Fig. 6f. For ethanol, acetone, n-butanol, 2-propanol, and methanol the β value is ≈0.87, 0.88, 0.83, 0.82, and 0.78, respectively, whereas the estimated detection limit for ethanol, acetone, n-butanol, 2-propanol, and methanol are ∼1, 0.8, 0.5, 0.4, 0.3 and 0.2 ppm, respectively.
Alloy NP-decorated thin film gas sensors as candidate materials for monitoring thermal runaway in Li-ion-batteries
From the gas sensing studies, it can be concluded that the surface decoration of the ZnO:Ag columnar films with AgAu NPs increases the selectivity to VOC vapors, while the surface decoration using AgPt NPs changes the response and leads to high selectivity to hydrogen gas. Thus, the selectivity of the ZnO:Ag films can be easily tuned using bimetallic NPs of different composition for surface decoration of the columns, which is of significant interest for gas sensing applications. In view of the versatility in tailoring the sensor's selectivity, and taking into account its fast response and recovery times, the alloy NP-decorated ZnO:Ag thin film sensors are highly promising materials for early hazard detection in LIBs, where many of the decomposition products, especially from the electrolyte solvents, are extremely flammable.25,27 Hazard detection in LIBs is especially demanding, as thermal runaway in a mal-functioning cell can rapidly destroy the whole device and other neighboring components, leading to a complete failure of all individual cells. For critical applications e.g. in the automotive sector, each cell should be equipped with a fast and robust hazard detection system in order to prevent fire spreading to other cells.In the case of thermal runaway, the LIB heats to over 200 °C and produces large amounts of H2 gas. Applying the alloy NP/ZnO:Ag thin film sensors in close vicinity of each battery cell would allow the identification of failing units as the H2 gas produced can be detected, whereas the thermal runaway provides the appropriate operating temperature for the sensor. Accordingly, the sensitivity of the AgPt/ZnO:Ag sensor towards H2 would be enhanced as the temperature is raised, making this decorated surface an excellent material for the detection of thermal runaway in LIB. In contrast, the AgAu/ZnO:Ag sensor has a significantly lower response towards H2 gas and is therefore an effective material to rule out sensor malfunction.
Thus, the integration of alloy NP/ZnO:Ag thin film sensors into individual battery cells is a potential pathway to achieve a cost effective and fast detection method of the early stages of thermal runaway, and to avoid the deterioration of other cells connected in parallel.
Gas sensing mechanism for bimetallic alloy NP-decorated films
The gas sensing mechanism of the decorated columnar films can be explained by changes in the electronic and chemical sensitization effects, i.e. the formation of nano-Schottky barriers at the interface between ZnO:Ag and the AgPt and AgAu alloy NPs,2,3,62 as well as the catalytic properties of the bimetallic NPs. Given that the diameter of the ZnO:Ag grains is larger than its Debye length, the nano-Schottky barriers formed at the Au/ZnO:Ag and Pt/ZnO:Ag interface have a low impact on the improvement of the gas sensing properties.10,63 Moreover, from the observation that ZnO:Ag columnar thin films decorated with AgPt and AgAu NPs exhibit very different sensing properties, it can be concluded that the chemical sensitization is the dominant effect (i.e. the catalytic properties of the bimetallic NPs). Choi et al. demonstrated that surface decoration of CuO NWs with different types of NPs, by modification of the conduction channel width, does not change the selectivity of the material.63 Therefore, the excellent catalytic properties of Pt towards the adsorption and dissociation of hydrogen is the key parameter that explains the change of selectivity by surface decoration with AgPt NPs.20 A number of studies have demonstrated that the addition of Pt NPs to different nano- and microstructures of metal oxides can greatly enhance the response to H2 gas.20,21,64–66 The Pt NPs may provide more active sites than any other noble metal for the adsorption and catalytic dissociation of oxygen species and H2 molecules.20,66 During the sensing process, the spillover and oxidation of hydrogen molecules by the adsorbed oxygen species (see eqn (1)) located near the AgPt bimetallic NPs, explains the high response to H2 gas.21,66 Taking into account the low concentration of roughly 12% of Pt in our AgPt bimetallic NPs, the strong shift of selectivity towards H2 gas is even more remarkable.| 2O−(ads) + 2H2 → 2H2O + 2e− | (1) |
Liu et al. also observed that the addition of Pt NPs to SnO2 leads to considerable decrease in the response to VOC vapors, while the addition of Au NPs induces the opposite effect.20,66 These findings also support the large effect of the chemical sensitization on the gas sensing properties and selectivity of the AgPt/ZnO:Ag columnar films towards H2 gas. Another mechanism which can explain the high selectivity of the AgPt/ZnO:Ag samples is the formation of PtHx species at the relatively high operating temperatures of 250–300 °C. Under these conditions, the number of hydrogen atoms per Pt site increases, and the metal becomes a better catalyst for hydride formation.21
For the AgAu/ZnO:Ag columnar films, the greatly enhanced sensing properties towards VOCs can be explained by the excellent catalytic properties of the Au component of the NPs towards the dissociation of oxygen and the (partial) oxidation of the VOC molecules.20,66–69 The Au NPs will provide more active sites for the adsorption of oxygen species than the pristine surface of ZnO.70 Many studies have demonstrated that the addition of Au NPs to nano- and microstructures of metal oxides leads to an enhancement in the overall response and selectivity to VOC vapors.67–71 The proposed reaction for the oxidation of the VOC molecules by the adsorbed oxygen species can be described as follows:11,70,72–74
| (Acetone) CH3COCH3 + 8O− ↔ 3CO2 + 3H2O + 8e− | (2) |
| (Ethanol) C2H5OH + 6O− ↔ 2CO2 + 3H2O + 6e− | (3) |
| (Methanol) CH3OH + O− ↔ HCHO + H2O + e− | (4) |
| (2-Propanol) C3H7OH + O− → (CH3)2CO + H2O + e− | (5) |
| (n-Butanol) C4H9OH + 12O− → 4CO2 + 5H2O + 12e− | (6) |
The high response to 2-propanol, compared to other VOC vapors, can be explained by the presence of methyne (CH) groups, which are essential for a more efficient decomposition and oxidation of this molecule.75,76 The general reaction can be described as:77
| VOC + nO− → aCO2 + bH2O + ne− | (7) |
Calculated properties
The DFT calculations have been performed to gain insight into the physio-chemical properties of the gas molecules (CH3CH2OH, CH3COCH3, n-C4H9OH, 2-C3H7OH, CH3OH and H2) adsorbed on the Ag-doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface decorated with Ag5Au5 and Ag9Pt clusters. The aim of these calculations is to support experiment and explain the observed enhanced sensitivity and the change in selectivity reported for the systems studied.
0) surface decorated with Ag5Au5 and Ag9Pt clusters. The aim of these calculations is to support experiment and explain the observed enhanced sensitivity and the change in selectivity reported for the systems studied.
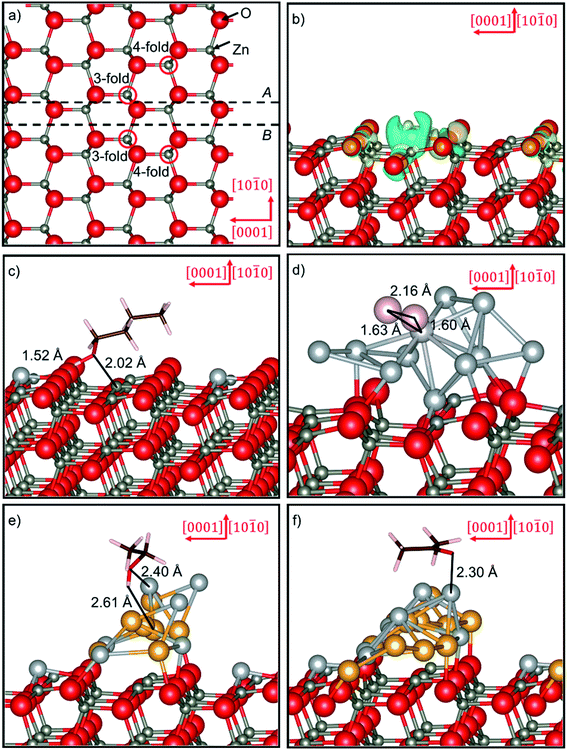
Terminations A and B of the ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface were modelled using slabs with an area of 98.574 Å2 and containing 96 atoms distributed in 8 atomic layers, see Fig. 7a. A vacuum gap of 20 Å along the direction perpendicular to the surface was added to separate each slab from its periodic image. More details regarding the stacking of the layers, and a description of the surface terminations and the number of layers with the atoms frozen at their bulk positions can be found elsewhere.49
0) surface were modelled using slabs with an area of 98.574 Å2 and containing 96 atoms distributed in 8 atomic layers, see Fig. 7a. A vacuum gap of 20 Å along the direction perpendicular to the surface was added to separate each slab from its periodic image. More details regarding the stacking of the layers, and a description of the surface terminations and the number of layers with the atoms frozen at their bulk positions can be found elsewhere.49
The substitutional doping of the ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface by Ag and its effect on the surface structure, energies, as well as on the electronic and magnetic properties are now examined. One Zn atom is replaced, in order to model the lowest Ag content value of 2.73 wt%, which is allowed by the size of our 48 formula unit (f.u.) computational cell. Despite modelling a dopant concentration almost threefold higher than the 0.95 wt% used for the experiments, previous works have shown excellent agreement between the two approaches.32,49 The substitutional doping of the symmetrically inequivalent exposed Zn atoms was inspected, corresponding to the following solid-state reaction,
0) surface by Ag and its effect on the surface structure, energies, as well as on the electronic and magnetic properties are now examined. One Zn atom is replaced, in order to model the lowest Ag content value of 2.73 wt%, which is allowed by the size of our 48 formula unit (f.u.) computational cell. Despite modelling a dopant concentration almost threefold higher than the 0.95 wt% used for the experiments, previous works have shown excellent agreement between the two approaches.32,49 The substitutional doping of the symmetrically inequivalent exposed Zn atoms was inspected, corresponding to the following solid-state reaction,
Zn48O48(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) + Ag(fcc) = Zn47AgO48(10 0) + Ag(fcc) = Zn47AgO48(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) + Zn(hcp) 0) + Zn(hcp) | (8) |
The surface free energy (σ) of the partially decorated material has been estimated using,
 | (9) |
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface, Edop is the energy of the Ag-doped ZnO(10
0) surface, Edop is the energy of the Ag-doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface, Epris is the energy of the pristine ZnO(10
0) surface, Epris is the energy of the pristine ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface, EAg is the energy of one atom in the bulk of fcc Ag and EZn is the energy of one atom in the bulk of hcp Zn. The γr values for the relaxed pristine ZnO(10
0) surface, EAg is the energy of one atom in the bulk of fcc Ag and EZn is the energy of one atom in the bulk of hcp Zn. The γr values for the relaxed pristine ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface were taken from previous work,12 and Table S2† shows the experimental and simulated lattice parameters for Ag and Zn.
0) surface were taken from previous work,12 and Table S2† shows the experimental and simulated lattice parameters for Ag and Zn.
Table 3 displays the surface free energies for the Ag-doped systems, indicating that only termination A is slightly stabilised by 14–18 meV Å−2 with respect to its pristine parent structure. However, substitutional doping of termination B, and in particular of its 3-fold Zn site, lead to the most thermodynamically stable doped surfaces, although they are less favourable than the pristine parent plane. The simulations suggest that in both terminations, doping the most exposed undercoordinated cation positions is ∼5 meV Å−2 more favourable than the substitution of the 4-fold sites. It was found that large in-plane displacements of approximately 1 Å for the atoms within the topmost layer account for the exothermic enthalpy during the insertion of Ag in termination A. Moreover, minor concomitant shifts of 0.15 Å towards the bulk are also observed for the sub-surface plane of Zn atoms and the surface layer of counter-anions. These horizontal and vertical atomic shifts are only allowed in the doped termination A due to the large interlayer spacing of 1.848 Å between the surface and sub-surface planes.49 In contrast, the densest topmost layers of termination B experience negligible displacements, with the exception of the O atoms surrounding the 3- and 4-fold Ag dopants, which move by 0.43 and 0.15 Å, respectively.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface.49 The surface free energies (σ) are also reported for both terminations of the Ag-doped ZnO(10
0) surface.49 The surface free energies (σ) are also reported for both terminations of the Ag-doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface. The work function (Φ) values are indicated for the pristine49 and doped surfaces
0) surface. The work function (Φ) values are indicated for the pristine49 and doped surfaces
| Termination | Doping site | γ r/σ (meV Å−2) | Φ (eV) |
|---|---|---|---|
| A | Pristine | 190 | 5.40 |
| 3-Fold | 172 | 2.50 | |
| 4-Fold | 176 | 2.61 | |
| B | Pristine | 84 | 5.80 |
| 3-Fold | 125 | 2.84 | |
| 4-Fold | 130 | 2.64 |
Bader charge analysis78–80 shows that Zn ions draw 58–60% of the electrons required to become neutral atoms from the Ag atoms, in agreement with their different charges in the pristine and doped surfaces, respectively. However, the largest electronic transfer is 68% when the 3-fold position of termination B is doped, which is also the most stable surface of this study. We found different redox compensation mechanisms for the electronic deficit caused by the replacement of the Zn atoms in the two terminations of the ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface. In termination A, the 3-fold Zn ions provide an excess of electrons, which also reduces the exposed oxygen atoms by a further 0.1e, thereby increasing the ionic character of the topmost surface layer. In termination B, the scenario for the charge compensation of Zn only involves the oxidation of its neighbouring oxygen atoms as illustrated in Fig. 7b. The charge density difference schemes were constructed by subtracting the sum of the electron charge densities of the clean surface and isolated dopant, with identical structure as in the doped form, from the electron density of the total system obtained from static calculations. The simulation of the doped terminations A and B suggests that most atoms remain non-magnetic following the process represented in eqn (8). However, the Ag introduced, alongside the 3-fold oxygen ions that directly coordinate it, become slightly magnetic at ca. 0.3 μB per atom. From the small magnetic moment calculated for the dopant, an electronic distribution of e2↑e2↓t3↑t3↓ has been inferred, corresponding to Ag+ ions in a pseudo-tetrahedral crystal field of oxygen atoms.
0) surface. In termination A, the 3-fold Zn ions provide an excess of electrons, which also reduces the exposed oxygen atoms by a further 0.1e, thereby increasing the ionic character of the topmost surface layer. In termination B, the scenario for the charge compensation of Zn only involves the oxidation of its neighbouring oxygen atoms as illustrated in Fig. 7b. The charge density difference schemes were constructed by subtracting the sum of the electron charge densities of the clean surface and isolated dopant, with identical structure as in the doped form, from the electron density of the total system obtained from static calculations. The simulation of the doped terminations A and B suggests that most atoms remain non-magnetic following the process represented in eqn (8). However, the Ag introduced, alongside the 3-fold oxygen ions that directly coordinate it, become slightly magnetic at ca. 0.3 μB per atom. From the small magnetic moment calculated for the dopant, an electronic distribution of e2↑e2↓t3↑t3↓ has been inferred, corresponding to Ag+ ions in a pseudo-tetrahedral crystal field of oxygen atoms.
The electronic properties of the doped surfaces were further interrogated by measuring the work function (Φ) in order to gauge their reactivity as reducing agents.32,49 The work function is the energy needed to remove an electron from the Fermi level (EF) of the surface into the electrostatic potential of the vacuum (VV), calculated as Φ = VV − EF. Table 3 shows large reductions of over 50% in the work function values of all the doped surfaces with respect to their pristine counterparts. The absolute value for this type of thermodynamic work is between 2.61 and 2.64 eV for the doped 4-fold position in both terminations. However, for further analysis, we focus on the 3-fold doped slab of termination B, which possesses the lowest surface energy and is therefore likely to be the prominent plane exposed in the crystal morphology of ZnO.
Impregnation with Ag5Au5 and Ag9Pt was also explored as a means to tune the sensing properties of the doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface and to reduce the detection limit of VOCs. Clusters composed of 10 atoms were chosen, as this is the smallest size to produce a close analogue to the experimental Ag content, i.e. 48 at% and 88 at% in AgAu and AgPt NPs, respectively (cf. Table S1†). The initial geometries for both noble bimetallic nanoclusters were derived from the fcc crystal structure common to the three monometallic materials81–83 and the secondary Au or Pt element was placed at random crystallographic positions. Table S2† illustrates the excellent agreement between the experimental and simulated lattice parameters for monometallic Au and Pt. Three different positions for the adsorption of the bimetallic clusters were tested, i.e. (i) above, (ii) close to, and (iii) far away from the Ag dopant. The term −(xEAg + yEX)/A was added to eqn (9), to evaluate the surface free energy of the fully decorated material; where X represents either fcc Au or Pt; EX is the energy of one such atom in the bulk; x and y are the numbers of Ag and X atoms, respectively, in the cluster; Edop becomes Edop+clus, which is the energy of the Ag-doped ZnO(10
0) surface and to reduce the detection limit of VOCs. Clusters composed of 10 atoms were chosen, as this is the smallest size to produce a close analogue to the experimental Ag content, i.e. 48 at% and 88 at% in AgAu and AgPt NPs, respectively (cf. Table S1†). The initial geometries for both noble bimetallic nanoclusters were derived from the fcc crystal structure common to the three monometallic materials81–83 and the secondary Au or Pt element was placed at random crystallographic positions. Table S2† illustrates the excellent agreement between the experimental and simulated lattice parameters for monometallic Au and Pt. Three different positions for the adsorption of the bimetallic clusters were tested, i.e. (i) above, (ii) close to, and (iii) far away from the Ag dopant. The term −(xEAg + yEX)/A was added to eqn (9), to evaluate the surface free energy of the fully decorated material; where X represents either fcc Au or Pt; EX is the energy of one such atom in the bulk; x and y are the numbers of Ag and X atoms, respectively, in the cluster; Edop becomes Edop+clus, which is the energy of the Ag-doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface decorated with the NPs; while the remaining variables keep their previous definition. The simulations suggest that the addition of the bimetallic clusters increases the surface free energy with respect to the doped surface, particularly for the Pt-based particles, see Table 4. The most thermodynamically favourable adsorption position depends on the nature of the cluster, as Ag5Au5 is likely to sit far from the Ag dopant, while Ag9Pt prefers a closer position.
0) surface decorated with the NPs; while the remaining variables keep their previous definition. The simulations suggest that the addition of the bimetallic clusters increases the surface free energy with respect to the doped surface, particularly for the Pt-based particles, see Table 4. The most thermodynamically favourable adsorption position depends on the nature of the cluster, as Ag5Au5 is likely to sit far from the Ag dopant, while Ag9Pt prefers a closer position.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface decorated with the Ag5Au5 and Ag9Pt clusters. The adsorption energy (Eads) for the NPs on the Ag-doped ZnO(10
0) surface decorated with the Ag5Au5 and Ag9Pt clusters. The adsorption energy (Eads) for the NPs on the Ag-doped ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface is reported. The relative position of the NP with respect to the Ag-dopant is also indicated. The doping was carried out in the 3-fold position of termination B
0) surface is reported. The relative position of the NP with respect to the Ag-dopant is also indicated. The doping was carried out in the 3-fold position of termination B
| Cluster | Position | σ (meV Å−2) | E ads (eV) | Φ (eV) |
|---|---|---|---|---|
| Ag5Au5 | Above | 181 | 5.54 | 3.01 |
| Close | 184 | 5.88 | 3.10 | |
| Far | 179 | 5.32 | 3.12 | |
| Ag9Pt | Above | 192 | 6.63 | 2.95 |
| Close | 209 | 8.30 | 2.95 | |
| Far | 194 | 6.79 | 3.15 |
The stability of the clusters on the surface was calculated using the adsorption energy (Eads):
| Eads = Edop+clus − Edop − xEAg − yEX | (10) |
The incorporation of both nanoclusters slightly raises the work function of the fully decorated surfaces with respect to the doped system. The lowest work function is predicted for the facet containing the Ag9Pt NP in the most favourable adsorption position. However, the largest value for this type of thermodynamic work was calculated for the surface decorated using the Ag5Au5 cluster in the most stable binding site. The small magnetic moments of the Ag dopant vanish after adsorption of the nanoclusters in any of the positions considered. Most of the adsorbed clusters are non-magnetic, but the simulations reveal a small mean value of 0.04 μB for each atom within the Ag5Au5 particles sited above and close to the dopant. Large positive charges of 1.4e− are located on the Ag atom of the cluster, while the secondary Au and Pt metals are reduced by −0.7 and −0.4e−, in agreement with their respective electronegativities. Upon adsorption, the net charge balance results in all noble bimetallic particles losing ∼0.9e− to the Zn atoms underneath and the O ions near the dopant.
The adsorption of the single molecules onto the doped and NP decorated ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surfaces was also studied and it was found that all these processes release energy, see Table 5. The molecules were introduced in several orientations at 1.5 Å from the surface and were subsequently allowed to relax to their equilibrium adsorption geometries. The calculated adsorption energies on the Ag-doped surface are roughly −1.5 to −1.7 eV for all the alcohols, which subtly depends directly on their molar mass. However, the strength of binding is approximately 0.5 eV less favourable for CH3COCH3 than for the alcohols, owing to the different chemical nature of their carbonyl and hydroxyl functional groups. The simulations suggest that the non-polar H2 molecule has the overall lowest adsorption energy at ∼−1.0 eV. The introduction of the Ag9Pt nanocluster reduces the energetic preference of the alcohols and ketone for the decorated surface by approximately 1.0 and 0.5 eV, respectively, compared to the doped system. The largest energy released at Eads = −1.387 eV was found for the adsorption of H2 on the Ag9Pt/ZnO:Ag surface, indicating a change in selectivity in agreement with our experiments. The impregnation with Ag5Au5 NPs leads to larger adsorption energies and enhanced sensitivity towards the VOCs with respect to the Ag9Pt/ZnO:Ag surface. Remarkably, the decreasing order of binding strength for the organic molecules is Eads(CH3COCH3) ≈ Eads(2-C3H7OH) ≈ Eads(C2H5OH) > Eads(n-C4H9OH) ≈ Eads(CH3OH), showing no dependence on the type of functional group. The binding energy of −0.070 eV for H2 on Ag5Au5/ZnO:Ag is the lowest calculated in this study, which explains the lack of selectivity found in our experiments for this molecule.
0) surfaces was also studied and it was found that all these processes release energy, see Table 5. The molecules were introduced in several orientations at 1.5 Å from the surface and were subsequently allowed to relax to their equilibrium adsorption geometries. The calculated adsorption energies on the Ag-doped surface are roughly −1.5 to −1.7 eV for all the alcohols, which subtly depends directly on their molar mass. However, the strength of binding is approximately 0.5 eV less favourable for CH3COCH3 than for the alcohols, owing to the different chemical nature of their carbonyl and hydroxyl functional groups. The simulations suggest that the non-polar H2 molecule has the overall lowest adsorption energy at ∼−1.0 eV. The introduction of the Ag9Pt nanocluster reduces the energetic preference of the alcohols and ketone for the decorated surface by approximately 1.0 and 0.5 eV, respectively, compared to the doped system. The largest energy released at Eads = −1.387 eV was found for the adsorption of H2 on the Ag9Pt/ZnO:Ag surface, indicating a change in selectivity in agreement with our experiments. The impregnation with Ag5Au5 NPs leads to larger adsorption energies and enhanced sensitivity towards the VOCs with respect to the Ag9Pt/ZnO:Ag surface. Remarkably, the decreasing order of binding strength for the organic molecules is Eads(CH3COCH3) ≈ Eads(2-C3H7OH) ≈ Eads(C2H5OH) > Eads(n-C4H9OH) ≈ Eads(CH3OH), showing no dependence on the type of functional group. The binding energy of −0.070 eV for H2 on Ag5Au5/ZnO:Ag is the lowest calculated in this study, which explains the lack of selectivity found in our experiments for this molecule.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surface and following decoration with the Ag5Au5 and Ag9Pt clusters. The relative position of the adsorbate with respect to the dopant is also indicated. The doping was carried out in the 3-fold position of termination B. Negative values of Δq indicate a charge transfer from the surface to the adsorbate
0) surface and following decoration with the Ag5Au5 and Ag9Pt clusters. The relative position of the adsorbate with respect to the dopant is also indicated. The doping was carried out in the 3-fold position of termination B. Negative values of Δq indicate a charge transfer from the surface to the adsorbate
| Molecule | Position | ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag 0):Ag |
Ag9Pt | Ag5Au5 | |||
|---|---|---|---|---|---|---|---|
| E ads (eV) | Δq (e−) | E ads (eV) | Δq (e−) | E ads (eV) | Δq (e−) | ||
| CH3CH2OH | Defect | −1.527 | 0.09 | −0.590 | −0.04 | −0.762 | 0.00 |
| Close | −1.486 | 0.02 | |||||
| Far | −1.602 | 0.03 | |||||
| CH3COCH3 | Defect | −1.122 | 0.07 | −0.663 | 0.02 | −0.798 | 0.00 |
| Close | −1.021 | 0.04 | |||||
| Far | −1.027 | 0.04 | |||||
| n-C4H9OH | Defect | −1.485 | 0.08 | −0.545 | 0.04 | −0.551 | −0.02 |
| Close | −1.632 | 0.03 | |||||
| Far | −1.706 | 0.03 | |||||
| 2-C3H7OH | Defect | −1.616 | 0.09 | −0.555 | 0.05 | −0.798 | 0.00 |
| Close | −1.530 | 0.03 | |||||
| Far | −1.597 | 0.03 | |||||
| CH3OH | Defect | −1.469 | 0.08 | −0.453 | −0.07 | −0.528 | −0.02 |
| Close | −1.425 | 0.02 | |||||
| Far | −1.456 | 0.02 | |||||
| H2 | Defect | −1.046 | 0.44 | −1.387 | −0.07 | −0.070 | −0.02 |
| Close | −0.268 | 0.02 | |||||
| Far | −0.291 | 0.03 | |||||
All the VOCs adsorb molecularly on the doped as well as on the NP-decorated ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surfaces, in line with previous reports.32,49 On the doped surface, three adsorption positions relative to the Ag atom were tested, i.e. the molecules directly interacting with the defect, or with its first or second nearest Zn neighbours. In the case of the VOCs, the preferred mode involves direct coordination between the adsorbate oxygen and the exposed Ag or Zn atom at around 2.15 and 2.00 Å, respectively. The hydroxy group of the alcohols forms a short hydrogen-bond at a distance of 1.39 to 1.53 Å with a surface 3-fold oxygen atom, as shown in Fig. 7c for n-C4H9OH. Other configurations are at least 0.108 eV less favourable than the most stable binding modes, as they involve dissociative adsorption or distorted hydrogen-bonds. For example, CH3OH can dissociate the hydroxy hydrogen, which is donated to the closest oxygen from the coordinated Ag adsorption site. Moreover, n-C4H9OH can form hydrogen-bonds with the oxygens lying either closer or farther away from the coordinated Ag and Zn cation than in the most stable adsorption modes. The free rotation around the C–OH bond and weak dispersion forces allow the hydrocarbon fragment of the alcohols to orientate as close as possible to the surface in all the adsorption modes. In contrast, the flat ketone molecule stays adsorbed in a canted configuration, owing to the tetrahedral nature of the metallic coordination site and the trigonal geometry of the sp2-hybridised carbonyl oxygen. Unsurprisingly, the H2 molecule has both minor exothermic molecular and moderately exothermic dissociative adsorption modes. This molecule appears tilted and hovering at 2.10 Å from any of the Zn sites and the closest 3-fold oxygen. H2 also dissociates upon interaction with the AgO or OO pair sites. For the dissociative adsorption configurations, the hydrogen atoms sit at 1.63 Å from the Ag-dopant and at the usual O–H bond distance of 0.98 Å from the 3-fold oxygen.
0) surfaces, in line with previous reports.32,49 On the doped surface, three adsorption positions relative to the Ag atom were tested, i.e. the molecules directly interacting with the defect, or with its first or second nearest Zn neighbours. In the case of the VOCs, the preferred mode involves direct coordination between the adsorbate oxygen and the exposed Ag or Zn atom at around 2.15 and 2.00 Å, respectively. The hydroxy group of the alcohols forms a short hydrogen-bond at a distance of 1.39 to 1.53 Å with a surface 3-fold oxygen atom, as shown in Fig. 7c for n-C4H9OH. Other configurations are at least 0.108 eV less favourable than the most stable binding modes, as they involve dissociative adsorption or distorted hydrogen-bonds. For example, CH3OH can dissociate the hydroxy hydrogen, which is donated to the closest oxygen from the coordinated Ag adsorption site. Moreover, n-C4H9OH can form hydrogen-bonds with the oxygens lying either closer or farther away from the coordinated Ag and Zn cation than in the most stable adsorption modes. The free rotation around the C–OH bond and weak dispersion forces allow the hydrocarbon fragment of the alcohols to orientate as close as possible to the surface in all the adsorption modes. In contrast, the flat ketone molecule stays adsorbed in a canted configuration, owing to the tetrahedral nature of the metallic coordination site and the trigonal geometry of the sp2-hybridised carbonyl oxygen. Unsurprisingly, the H2 molecule has both minor exothermic molecular and moderately exothermic dissociative adsorption modes. This molecule appears tilted and hovering at 2.10 Å from any of the Zn sites and the closest 3-fold oxygen. H2 also dissociates upon interaction with the AgO or OO pair sites. For the dissociative adsorption configurations, the hydrogen atoms sit at 1.63 Å from the Ag-dopant and at the usual O–H bond distance of 0.98 Å from the 3-fold oxygen.
The addition of Ag9Pt changes the adsorption geometry of CH3OH, which prefers to interact through its hydroxy H with the Pt at a distance of 2.30 Å, which increases by 0.38 Å for C2H5OH. However, the alcohols of higher molar mass, i.e. n-C4H9OH and 2-C3H7OH, instead coordinate to the Pt atom by their hydroxy O at an average distance of 2.37 Å. CH3COCH3 binds the Pt atom at 2.20 Å, which is the smallest value reported for any VOC interacting with the Ag9Pt/ZnO:Ag surface. Different behaviours were calculated for the hydrocarbon radical of the VOCs, which lies more parallel to the surface for the low mass alcohols and the ketone than for n-C4H9OH and 2-C3H7OH. H2 dissociates upon adsorption to the Pt site, with the H atoms separated by 2.16 Å, explaining the largest exothermic enthalpy for the interaction with the supported Ag9Pt cluster, as shown in Fig. 7d. Shifting to the Ag5Au5 particle increases the number of interactions between the surface and the VOC molecules, justifying the larger adsorption energies with respect to the Ag9Pt/ZnO:Ag surface. The DFT adsorption geometries indicate that 2-C3H7OH and C2H5OH form the dative O–Ag and H–Au bonds at 2.40 and 2.62 Å, respectively, see Fig. 7e. Furthermore, n-C4H9OH and CH3OH have similar adsorption configurations as the alcohols of intermediate molecular mass, but coordinate the Au atom at least 0.14 Å further away. CH3COCH3 can only form the O–Ag interaction at 2.30 Å, which is the shortest for the bimetallic Ag5Au5 cluster, as displayed in Fig. 7f. Finally, the H2 molecule stays perpendicular to the surface and 2.92 Å from the Au atom, leading to the smallest adsorption energy calculated for this molecule in this study.
Bader charge analysis shows that all VOCs become positively charged upon adsorption on the doped surface, with the largest transfer of 0.09e− obtained for the Ag site, as shown in Table 5. Charge donations decrease around 50% after introducing the Ag9Pt cluster and the negative values calculated for the low molecular mass alcohols confirm their adsorption geometry via the hydroxy H. Electron transfers vanish for the molecules adsorbed on Ag5Au5, with the exception of the negatively charged n-C4H9OH and CH3OH, which sit furthest away from the cluster. H2 suffers the largest charge withdrawal reported for adsorptions on the doped surface but it is predicted that the H–Zn hydrogen gains −0.20e− while the hydroxy H loses 0.64e−. The reverse direction of electron transport for the interaction with the bimetallic nanoclusters was observed, as H2 gains small electronic charge and remains molecular.
We believe that the change in selectivity is the result of adding Pt, which displays excellent catalytic properties for binding and dissociating the H2 molecule, which was discussed in the section “Gas sensing mechanism for bimetallic alloy NP-decorated films”. The computational simulations that we performed provide further insight into the sensing mechanism at the molecular level. We have studied the adsorption of VOCs and H2 onto the surfaces of the pristine ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag surface and after impregnation of the alloy nanoparticles. Upon adsorption, the VOCs release the largest adsorption energies on the pristine ZnO(10
0):Ag surface and after impregnation of the alloy nanoparticles. Upon adsorption, the VOCs release the largest adsorption energies on the pristine ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag facet. The pristine surface contains the largest proportion of positively charged Zn cations exposed, which are available to coordinate the O atoms of the VOCs. As a result, the H2 and VOC molecules donate charge to the surface and become positively charged, see Table 5. However, the introduction of the clusters reduces the number of accessible surface Zn atoms, decreasing considerably the adsorption energy and charge transfers of the VOCs, which in many cases experience a net electron gain. In particular, the Pt atom of the Ag9Pt nanoparticle is able to dissociate catalytically the H2 molecule, which displays the largest adsorption energy in comparison to the VOCs. For the surfaces decorated with the Ag5Au5 NPS, we calculated the largest difference of adsorption energy between VOCs and H2 molecule. This enhanced sensitivity compared to the pristine surface can be rationalized in terms of the number of interactions that the VOC molecules are able form with the Au atoms in the Ag5Au5 clusters. Thus, the computational results are in agreement, complement and explain the changes in selectivity and sensitivity observed experimentally.
0):Ag facet. The pristine surface contains the largest proportion of positively charged Zn cations exposed, which are available to coordinate the O atoms of the VOCs. As a result, the H2 and VOC molecules donate charge to the surface and become positively charged, see Table 5. However, the introduction of the clusters reduces the number of accessible surface Zn atoms, decreasing considerably the adsorption energy and charge transfers of the VOCs, which in many cases experience a net electron gain. In particular, the Pt atom of the Ag9Pt nanoparticle is able to dissociate catalytically the H2 molecule, which displays the largest adsorption energy in comparison to the VOCs. For the surfaces decorated with the Ag5Au5 NPS, we calculated the largest difference of adsorption energy between VOCs and H2 molecule. This enhanced sensitivity compared to the pristine surface can be rationalized in terms of the number of interactions that the VOC molecules are able form with the Au atoms in the Ag5Au5 clusters. Thus, the computational results are in agreement, complement and explain the changes in selectivity and sensitivity observed experimentally.
Conclusions
In summary, the surface of ZnO:Ag nanostructured columnar grains was successfully decorated with AgPt and AgAu bimetallic noble bimetallic alloy NPs using a custom-made high vacuum deposition system with an in-house Haberland type gas aggregation source. Gas sensing measurements were carried out to reveal the response of the thin films to H2 gas and various VOC vapors. The underlying gas sensing mechanisms for the AgPt as well as AgAu NP-decorated ZnO:Ag thin films was proposed and discussed in terms of the electronic and chemical sensitization effect of the bimetallic alloy NPs.The nanocomposites with AgAu NPs exhibit a highly improved response to VOC vapors compared to the pristine ZnO:Ag thin films and previously reported nanocomposites based on ZnO:Ag and Ag NPs. The gas response to 100 ppm of ethanol, acetone, n-butanol, 2-propanol and methanol vapors was increased by a factor of ∼2.8, ∼6, ∼3.2, ∼3 and ∼4.5, respectively, compared to pristine ZnO:Ag thin films. In contrast, the nanocomposite decorated with the AgPt NPs shows a remarkable change in selectivity to H2 gas. While the response to all VOC vapors is considerably decreased once AgPt NPs are introduced on the surface of ZnO:Ag columnar films, the response toward H2 is increased by more than one order of magnitude.
The affinity of small molecules towards the doped and NP decorated ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surfaces was also investigated computationally. Doping with Ag atoms was modelled, where it was found that the most stable substitution occurs for the 3-fold Zn surface sites. Subsequently, Ag9Pt and Ag5Au5 clusters prefer to bind the surface above and far away from the dopant atom, respectively. When studying the binding of CH3CH2OH, CH3COCH3, n-C4H9OH, 2-C3H7OH, CH3OH and H2 on the doped surfaces and at the nanoclusters, it was found that the VOCs release the largest and smallest energies upon adsorption to the doped surfaces and Ag9Pt cluster, respectively, in agreement with the enhanced selectivity of the former. The dissociative adsorption of H2 on the Ag9Pt/ZnO(10
0) surfaces was also investigated computationally. Doping with Ag atoms was modelled, where it was found that the most stable substitution occurs for the 3-fold Zn surface sites. Subsequently, Ag9Pt and Ag5Au5 clusters prefer to bind the surface above and far away from the dopant atom, respectively. When studying the binding of CH3CH2OH, CH3COCH3, n-C4H9OH, 2-C3H7OH, CH3OH and H2 on the doped surfaces and at the nanoclusters, it was found that the VOCs release the largest and smallest energies upon adsorption to the doped surfaces and Ag9Pt cluster, respectively, in agreement with the enhanced selectivity of the former. The dissociative adsorption of H2 on the Ag9Pt/ZnO(10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0):Ag surface causes the largest calculated adsorption energy for any of the NP-decorated surfaces, supporting the change in selectivity reported for this system.
0):Ag surface causes the largest calculated adsorption energy for any of the NP-decorated surfaces, supporting the change in selectivity reported for this system.
We have shown that the decoration of ZnO:Ag columnar thin films with noble bimetallic NPs provides a viable route to tailor the sensitivity as well as selectivity of ZnO:Ag gas sensors. It was found that AgAu NPs lead to high sensitivity as well as fast response and recovery times for the detection of the VOC types studied. In addition, the possibility to change the selectivity of ZnO:Ag thin films by surface decoration with bimetallic NPs is a feasible, effective and flexible approach for the fabrication of highly selective sensors.
Considering the low concentration of about 12 at% of Pt in the AgPt bimetallic alloy NPs, this sensor is a promising prototype of a cost effective H2 gas sensor for early hazard detection in lithium ion batteries. Given the high versatility and adaptability of the thin film fabrication process, in combination with the application of alloy NPs for surface decoration, the approach presented in this work offers significant potential for gas sensing applications, for example for the early detection of thermal runaway in lithium ion batteries LIB.
Author contributions
A. V., V. P., and O. L. synthesized the ZnO:Ag nanomaterial functionalized using AgAu and AgPt bimetallic alloy NPs. O. L. developed the synthesis methodology from chemical solution (SCS) for ZnO. A. V. and F. F. developed the functionalization procedure for the AgAu and AgPt bimetallic alloy NPs, and set-up all experiments and XPS analysis. O. L., V. P., A. V., M. I. T., M. H., H. C. and S. N. adapted a technological approach for material synthesis and integration/fabrication of the sensors for early hazard detection in batteries. V. P. and O. L. carried out the measurement of the sensing properties of the thin film materials and analyzed the data. T. D., N. W. and L. K. performed the TEM studies and analysis. V. P., O. L., S. N., R. A., M. I. T. and H. C. analyzed the results, including the experimental data and the revised draft. N. H. d. L., D. S. C. and A. C. E. realized the computational part and revised draft. D. S. C. analysed the computational results. A. V., P. V., D. S. C., S. N., T. D., N. W. and O. L. prepared the manuscript draft. O. L., A. V., F. F., L. K., S. N. and R. A. conducted the study conception and design, and gave the final approval of the manuscript version to be published. The manuscript was written through contributions of all authors. All authors reviewed the manuscript. Katrin Brandenburg is acknowledged for her help in the final proof-reading of the manuscript.Data availability
All data created during this research is openly available from Cardiff University's Research Portal at http://www.doi.org/10.17035/d.2019.0081500252.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Lupan acknowledges the Alexander von Humboldt Foundation for the research fellowship for experienced researchers 3-3MOL/1148833 STP at the Institute for Materials Science, Kiel University, Germany. This research was funded partially by the German Research Foundation (DFG) under schemes SFB1261 and FOR2093 ‘Memristive devices for neuronal systems’ as well as by the Federal Ministry of Education and Research in Germany under project “PorSSi” (03XP0126 B). Dr O. Polonskyi is acknowledged for fruitful discussions of XPS part. This research was sponsored in part by the NATO Science for Peace and Security Programme (SPS) under grant G5634 “Advanced Electro-Optical Chemical Sensors” AMOXES. N. H. d. L., D. S. C. and A. C. E. acknowledge the Engineering and Physical Sciences Research Council (EPSRC grant EP/K009567/2) for funding. Via their membership of the UK's HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202/1, EP/R029431/1), this work used the ARCHER UK National Supercomputing Service (https://www.archer.ac.uk). This work also employed the computational facilities of the Advanced Research Computing @ Cardiff (ARCCA) Division, Cardiff University and the Supercomputing Facilities at Cardiff University operated by ARCCA on behalf of the Cardiff Supercomputing Facility and the Supercomputing Wales (SCW) projects. We acknowledge the support of the latter, which is part-funded by the European Regional Development Fund (ERDF) via Welsh Government. The authors thank Salih Veziroglu for his contribution to the design and the graphic illustration of the cover graphic.References
- O. Lupan, V. Postica, F. Labat, I. Ciofini, T. Pauporté and R. Adelung, Ultra-Sensitive and Selective Hydrogen Nanosensor With Fast Response at Room Temperature Based on a Single Pd/ZnO Nanowire, Sens. Actuators, B, 2018, 254, 1259–1270 CrossRef CAS.
- A. Kolmakov, D. O. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Enhanced Gas Sensing by Individual SnO2 Nanowires and Nanobelts Functionalized with Pd Catalyst Particles, Nano Lett., 2005, 5, 667–673 CrossRef CAS PubMed.
- N. Yamazoe, New approaches for improving semiconductor gas sensors, Sens. Actuators, B, 1991, 5, 7–19 CrossRef CAS.
- S. Bano, N. Ahmad, S. Sultana, S. Sabir and M. Z. Khan, Preparation and study of ternary polypyrrole-tin oxide-chitin nanocomposites and their potential applications in visible light photocatalysis and sensors, J. Environ. Chem. Eng., 2019, 7, 103012 CrossRef CAS.
- H. Tian, H. Fan, J. Ma, Z. Liu, L. Ma, S. Lei, J. Fang and C. Long, Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing, J. Hazard. Mater., 2018, 341, 102–111 CrossRef PubMed.
- L. Ma, H. Fan, H. Tian, J. Fang and X. Qian, The n-ZnO/n-In2O3 heterojunction formed by a surface-modification and their potential barrier-control in methanal gas sensing, Sens. Actuators, B, 2016, 222, 508–516 CrossRef CAS.
- J. Li, H. Fan and X. Jia, Multilayered ZnO Nanosheets with 3D Porous Architectures: Synthesis and Gas Sensing Application, J. Phys. Chem. C, 2010, 114, 14684–14691 CrossRef CAS.
- H. Tian, H. Fan, J. Ma, L. Ma and G. Dong, Noble metal-free modified electrode of exfoliated graphitic carbon nitride/ZnO nanosheets for highly efficient hydrogen peroxide sensing, Electrochim. Acta, 2017, 247, 787–794 CrossRef CAS.
- S.-S. Chen, X.-X. Lin, A.-J. Wang, H. Huang and J.-J. Feng, Facile synthesis of multi-branched AgPt alloyed nanoflowers and their excellent applications in surface enhanced Raman scattering, Sens. Actuators, B, 2017, 248, 214–222 CrossRef CAS.
- O. Lupan, V. Postica, R. Adelung, F. Labat, I. Ciofini, U. Schürmann, L. Kienle, L. Chow, B. Viana and T. Pauporté, Functionalized Pd/ZnO Nanowires for Nanosensors, Phys. Status Solidi RRL, 2017, 12, 1700321 CrossRef.
- J. Guo, J. Zhang, M. Zhu, D. Ju, H. Xu and B. Cao, High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles, Sens. Actuators, B, 2014, 199, 339–345 CrossRef CAS.
- S. M. Majhi, P. Rai and Y.-T. Yu, Facile Approach to Synthesize Au@ZnO Core–Shell Nanoparticles and Their Application for Highly Sensitive and Selective Gas Sensors, ACS Appl. Mater. Interfaces, 2015, 7, 9462–9468 CrossRef CAS PubMed.
- O. Lupan, V. Postica, N. Wolff, J. Su, F. Labat, I. Ciofini, H. Cavers, R. Adelung, O. Polonskyi, F. Faupel, L. Kienle, B. Viana and T. Pauporté, Low-Temperature Solution Synthesis of Au-Modified ZnO Nanowires for Highly Efficient Hydrogen Nanosensors, ACS Appl. Mater. Interfaces, 2019, 11, 32115–32126 CrossRef CAS PubMed.
- A. Shafi, N. Ahmad, S. Sultana, S. Sabir and M. Z. Khan, Ag2S-Sensitized NiO–ZnO Heterostructures with Enhanced Visible Light Photocatalytic Activity and Acetone Sensing Property, ACS Omega, 2019, 4, 12905–12918 CrossRef CAS PubMed.
- J. P. V. Damasceno, C. M. Maroneze, M. Strauss, F. A. Sigoli and I. O. Mazali, Preparation of supported AuPd nanoalloys mediated by ionic liquid-like functionalized SBA-15: structural correlations concerning its catalytic activity, New J. Chem., 2016, 40, 6636–6642 RSC.
- Y. Yong, C. Li, X. Li, T. Li, H. Cui and S. Lv, Ag7Au6 Cluster as a Potential Gas Sensor for CO, HCN, and NO Detection, J. Phys. Chem. C, 2015, 119, 7534–7540 CrossRef CAS.
- S.-J. Kim, S.-J. Choi, J.-S. Jang, H.-J. Cho, W.-T. Koo, H. L. Tuller and I.-D. Kim, Exceptional High-Performance of Pt-Based Bimetallic Catalysts for Exclusive Detection of Exhaled Biomarkers, Adv. Mater., 2017, 29, 1700737 CrossRef PubMed.
- M. M. Rahman, S. B. Khan, A. M. Asiri, K. A. Alamry, A. A. P. Khan, A. Khan, M. A. Rub and N. Azum, Acetone sensor based on solvothermally prepared ZnO doped with Co3O4 nanorods, Microchim. Acta, 2013, 180, 675–685 CrossRef CAS PubMed.
- S.-W. Choi, A. Katoch, G.-J. Sun and S. S. Kim, Bimetallic Pd/Pt nanoparticle-functionalized SnO2 nanowires for fast response and recovery to NO2, Sens. Actuators, B, 2013, 181, 446–453 CrossRef CAS.
- F. Fan, J. Zhang, J. Li, N. Zhang, R. Hong, X. Deng, P. Tang and D. Li, Hydrogen sensing properties of Pt-Au bimetallic nanoparticles loaded on ZnO nanorods, Sens. Actuators, B, 2017, 241, 895–903 CrossRef CAS.
- K. Hassan and G.-S. Chung, Catalytically activated quantum-size Pt/Pd bimetallic core–shell nanoparticles decorated on ZnO nanorod clusters for accelerated hydrogen gas detection, Sens. Actuators, B, 2017, 239, 824–833 CrossRef CAS.
- T. Shiravand and A. Azadbakht, Impedimetric biosensor based on bimetallic AgPt nanoparticle-decorated carbon nanotubes as highly conductive film surface, J. Solid State Electrochem., 2017, 21, 1699–1711 CrossRef CAS.
- K. K. Haldar, S. Kundu and A. Patra, Core-Size-Dependent Catalytic Properties of Bimetallic Au/Ag Core–Shell Nanoparticles, ACS Appl. Mater. Interfaces, 2014, 6, 21946–21953 CrossRef CAS PubMed.
- Z. Y. Li, J. P. Wilcoxon, F. Yin, Y. Chen, R. E. Palmer and R. L. Johnston, Structures and optical properties of 4-5 nm bimetallic AgAu nanoparticles, Faraday Discuss., 2008, 138, 363–373 RSC.
- M. Metzger, B. Strehle, S. Solchenbach and H. A. Gasteiger, Origin of H2 Evolution in LIBs: H2O Reduction vs. Electrolyte Oxidation, J. Electrochem. Soc., 2016, 163, A798–A809 CrossRef CAS.
- D. Ortiz, V. Steinmetz, D. Durand, S. Legand, V. Dauvois, P. Maître and S. Le Caër, Radiolysis as a solution for accelerated ageing studies of electrolytes in lithium-ion batteries, Nat. Commun., 2015, 6, 6950 CrossRef PubMed.
- A. W. Golubkov, D. Fuchs, J. Wagner, H. Wiltsche, C. Stangl, G. Fauler, G. Voitic, A. Thaler and V. Hacker, Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes, RSC Adv., 2014, 4, 3633–3642 RSC.
- V. Postica, I. Hölken, V. Schneider, V. Kaidas, O. Polonskyi, V. Cretu, I. Tiginyanu, F. Faupel, R. Adelung and O. Lupan, Multifunctional device based on ZnO:Fe nanostructured films with enhanced UV and ultra-fast ethanol vapour sensing, Mater. Sci. Semicond. Process., 2016, 49, 20–33 CrossRef CAS.
- J. Gröttrup, V. Postica, N. Ababii, O. Lupan, C. Zamponi, D. Meyners, Y. K. Mishra, V. Sontea, I. Tiginyanu and R. Adelung, Size-Dependent UV and Gas Sensing Response of Individual Fe2O3-ZnO:Fe Micro- and Nanowire Based Devices, J. Alloys Compd., 2017, 701, 920–925 CrossRef.
- O. Lupan, L. Chow, S. Shishiyanu, E. Monaico, T. Shishiyanu, V. Şontea, B. Roldan Cuenya, A. Naitabdi, S. Park and A. Schulte, Nanostructured zinc oxide films synthesized by successive chemical solution deposition for gas sensor applications, Mater. Res. Bull., 2009, 44, 63–69 CrossRef CAS.
- O. Lupan, S. Shishiyanu, V. Ursaki, H. Khallaf, L. Chow, T. Shishiyanu, V. Sontea, E. Monaico and S. Railean, Synthesis of nanostructured Al-doped zinc oxide films on Si for solar cells applications, Sol. Energy Mater. Sol. Cells, 2009, 93, 1417–1422 CrossRef CAS.
- V. Postica, A. Vahl, D. Santos-Carballal, T. Dankwort, L. Kienle, M. Hoppe, A. Cadi-Essadek, N. H. de Leeuw, M.-I. Terasa, R. Adelung, F. Faupel and O. Lupan, Tuning ZnO Sensors Reactivity toward Volatile Organic Compounds via Ag Doping and Nanoparticle Functionalization, ACS Appl. Mater. Interfaces, 2019, 11, 31452–31466 CrossRef CAS PubMed.
- O. Polonskyi, T. Peter, A. M. Ahadi, A. Hinz, T. Strunskus, V. Zaporojtchenko, H. Biederman and F. Faupel, Huge increase in gas phase nanoparticle generation by pulsed direct current sputtering in a reactive gas admixture, Appl. Phys. Lett., 2013, 103, 033118 CrossRef.
- H. Haberland, M. Karrais, M. Mall and Y. Thurner, Thin films from energetic cluster impact: a feasibility study, J. Vac. Sci. Technol., A, 1992, 10, 3266–3271 CrossRef CAS.
- P. Solař, O. Polonskyi, A. Olbricht, A. Hinz, A. Shelemin, O. Kylián, A. Choukourov, F. Faupel and H. Biederman, Single-step generation of metal-plasma polymer multicore@shell nanoparticles from the gas phase, Sci. Rep., 2017, 7, 8514 CrossRef PubMed.
- A. Vahl, J. Strobel, W. Reichstein, O. Polonskyi, T. Strunskus, L. Kienle and F. Faupel, Single target sputter deposition of alloy nanoparticles with adjustable composition via a gas aggregation cluster source, Nanotechnology, 2017, 28, 175703 CrossRef PubMed.
- O. Lupan, V. Postica, M. Mecklenburg, K. Schulte, Y. K. Mishra, B. Fiedler and R. Adelung, Low powered, tunable and ultra-light aerographite sensor for climate relevant gas monitoring, J. Mater. Chem. A, 2016, 4, 16723–16730 RSC.
- O. Lupan, V. Cretu, V. Postica, N. Ababii, O. Polonskyi, V. Kaidas, F. Schütt, Y. K. Mishra, E. Monaico, I. Tiginyanu, V. Sontea, T. Strunskus, F. Faupel and R. Adelung, Enhanced ethanol vapour sensing performances of copper oxide nanocrystals with mixed phases, Sens. Actuators, B, 2016, 224, 434–448 CrossRef CAS.
- V. Cretu, V. Postica, A. K. Mishra, M. Hoppe, I. Tiginyanu, Y. K. Mishra, L. Chow, N. H. de Leeuw, R. Adelung and O. Lupan, Synthesis, characterization and DFT studies of zinc-doped copper oxide nanocrystals for gas sensing applications, J. Mater. Chem. A, 2016, 4, 6527–6539 RSC.
- G. Kresse and J. Hafner, Ab initio molecular dynamics for liquid metals, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Ab initio molecular-dynamics simulation of the liquid-metal--amorphous-semiconductor transition in germanium, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- V. Postica, A. Vahl, J. Strobel, D. Santos-Carballal, O. Lupan, A. Cadi-Essadek, N. H. de Leeuw, F. Schütt, O. Polonskyi, T. Strunskus, M. Baum, L. Kienle, R. Adelung and F. Faupel, Tuning doping and surface functionalization of columnar oxide films for volatile organic compound sensing: experiments and theory, J. Mater. Chem. A, 2018, 6, 23669–23682 RSC.
- V. I. Anisimov, M. A. Korotin, J. Zaanen and O. K. Andersen, Spin bags, polarons, and impurity potentials in La2-xSrxCuO4 from first principles, Phys. Rev. Lett., 1992, 68, 345–348 CrossRef CAS PubMed.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- D. O. Scanlon, B. J. Morgan and G. W. Watson, The origin of the enhanced oxygen storage capacity of Ce1−x(Pd/Pt)xO2, Phys. Chem. Chem. Phys., 2011, 13, 4279–4284 RSC.
- H. J. Monkhorst and J. D. Pack, Special points for Brillouin-zone integrations, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- N. D. Mermin, Thermal Properties of the Inhomogeneous Electron Gas, Phys. Rev., 1965, 137, A1441–A1443 CrossRef.
- H. Okamoto, Ag-Pt (silver-platinum), J. Phase Equilib., 1997, 18, 485 CrossRef CAS.
- G. L. W. Hart, L. J. Nelson, R. R. Vanfleet, B. J. Campbell, M. H. F. Sluiter, J. H. Neethling, E. J. Olivier, S. Allies, C. I. Lang, B. Meredig and C. Wolverton, Revisiting the revised Ag-Pt phase diagram, Acta Mater., 2017, 124, 325–332 CrossRef CAS.
- H. Tian, H. Fan, M. Li and L. Ma, Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor, ACS Sens., 2016, 1, 243–250 CrossRef CAS.
- I.-S. Hwang, S.-J. Kim, J.-K. Choi, J.-J. Jung, D. J. Yoo, K.-Y. Dong, B.-K. Ju and J.-H. Lee, Large-scale fabrication of highly sensitive SnO2 nanowire network gas sensors by single step vapor phase growth, Sens. Actuators, B, 2012, 165, 97–103 CrossRef CAS.
- J. J. Hassan, M. A. Mahdi, C. W. Chin, H. Abu-Hassan and Z. Hassan, A high-sensitivity room-temperature hydrogen gas sensor based on oblique and vertical ZnO nanorod arrays, Sens. Actuators, B, 2013, 176, 360–367 CrossRef CAS.
- A. Katoch, S.-W. Choi, H. W. Kim and S. S. Kim, Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism, J. Hazard. Mater., 2015, 286, 229–235 CrossRef CAS PubMed.
- A. Katoch, Z. U. Abideen, H. W. Kim and S. S. Kim, Grain-Size-Tuned Highly H2-Selective Chemiresistive Sensors Based on ZnO–SnO2 Composite Nanofibers, ACS Appl. Mater. Interfaces, 2016, 8, 2486–2494 CrossRef CAS PubMed.
- R.-J. Wu, D.-J. Lin, M.-R. Yu, M. H. Chen and H.-F. Lai, Ag@SnO2 core–shell material for use in fast-response ethanol sensor at room operating temperature, Sens. Actuators, B, 2013, 178, 185–191 CrossRef CAS.
- S.-W. Choi, A. Katoch, J.-H. Kim and S. S. Kim, Remarkable Improvement of Gas-Sensing Abilities in p-type Oxide Nanowires by Local Modification of the Hole-Accumulation Layer, ACS Appl. Mater. Interfaces, 2015, 7, 647–652 CrossRef CAS PubMed.
- C. S. Rout, A. R. Raju, A. Govindaraj and C. N. R. Rao, Hydrogen sensors based on ZnO nanoparticles, Solid State Commun., 2006, 138, 136–138 CrossRef CAS.
- L. F. Zhu, J. C. She, J. Y. Luo, S. Z. Deng, J. Chen and N. S. Xu, Study of Physical and Chemical Processes of H2 Sensing of Pt-Coated WO3 Nanowire Films, J. Phys. Chem. C, 2010, 114, 15504–15509 CrossRef CAS.
- C. Liu, Q. Kuang, Z. Xie and L. Zheng, The effect of noble metal (Au, Pd and Pt) nanoparticles on the gas sensing performance of SnO2-based sensors: a case study on the {221} high-index faceted SnO2 octahedra, CrystEngComm, 2015, 17, 6308–6313 RSC.
- W. Xia, C. Mei, X. Zeng, G. Fan, J. Lu, X. Meng and X. Shen, Nanoplate-Built ZnO Hollow Microspheres Decorated with Gold Nanoparticles and Their Enhanced Photocatalytic and Gas-Sensing Properties, ACS Appl. Mater. Interfaces, 2015, 7, 11824–11832 CrossRef CAS PubMed.
- A. L. Zou, Y. Qiu, J. J. Yu, B. Yin, G. Y. Cao, H. Q. Zhang and L. Z. Hu, Ethanol sensing with Au-modified ZnO microwires, Sens. Actuators, B, 2016, 227, 65–72 CrossRef CAS.
- X.-j. Wang, W. Wang and Y.-L. Liu, Enhanced acetone sensing performance of Au nanoparticles functionalized flower-like ZnO, Sens. Actuators, B, 2012, 168, 39–45 CrossRef CAS.
- X. Li, X. Zhou, H. Guo, C. Wang, J. Liu, P. Sun, F. Liu and G. Lu, Design of Au@ZnO Yolk–Shell Nanospheres with Enhanced Gas Sensing Properties, ACS Appl. Mater. Interfaces, 2014, 6, 18661–18667 CrossRef CAS PubMed.
- X. Liu, J. Zhang, L. Wang, T. Yang, X. Guo, S. Wu and S. Wang, 3D hierarchically porous ZnO structures and their functionalization by Au nanoparticles for gas sensors, J. Mater. Chem., 2011, 21, 349–356 RSC.
- W. Tang, J. Wang, P. Yao and X. Li, Hollow hierarchical SnO2-ZnO composite nanofibers with heterostructure based on electrospinning method for detecting methanol, Sens. Actuators, B, 2014, 192, 543–549 CrossRef CAS.
- S. Wlodek, K. Colbow and F. Consadori, Signal-shape analysis of a thermally cycled tin-oxide gas sensor, Sens. Actuators, B, 1991, 3, 63–68 CrossRef CAS.
- Y. Wang, B. Zhang, J. Liu, Q. Yang, X. Cui, Y. Gao, X. Chuai, F. Liu, P. Sun, X. Liang, Y. Sun and G. Lu, Au-loaded mesoporous WO3: preparation and n-butanol sensing performances, Sens. Actuators, B, 2016, 236, 67–76 CrossRef CAS.
- K. Arshak and I. Gaidan, Development of a novel gas sensor based on oxide thick films, Mater. Sci. Eng., B, 2005, 118, 44–49 CrossRef.
- M. Rafiqul Islam, N. Kumazawa and M. Takeuchi, Titaniumdioxide chemical sensor working with AC voltage, Sens. Actuators, B, 1998, 46, 114–119 CrossRef.
- Z. Feng, Y. Ma, V. Natarajan, Q. Zhao, X. Ma and J. Zhan, In-situ generation of highly dispersed Au nanoparticles on porous ZnO nanoplates via ion exchange from hydrozincite for VOCs gas sensing, Sens. Actuators, B, 2018, 255, 884–890 CrossRef CAS.
- G. Henkelman, A. Arnaldsson and H. Jónsson, A fast and robust algorithm for Bader decomposition of charge density, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- E. Sanville, S. D. Kenny, R. Smith and G. Henkelman, Improved grid-based algorithm for Bader charge allocation, J. Comput. Chem., 2007, 28, 899–908 CrossRef CAS PubMed.
- W. Tang, E. Sanville and G. Henkelman, A grid-based Bader analysis algorithm without lattice bias, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS PubMed.
- K. H. Hong, G. M. McNally, M. Coduri and J. P. Attfield, Synthesis, Crystal Structure, and Magnetic Properties of MnFe3O5, J. Inorg. Gen. Chem., 2016, 642, 1355–1358 CAS.
- L. P. Salamakha, E. Bauer, S. I. Mudryi, A. P. Gonçalves, M. Almeida and H. Noël, Isothermal section of the Ce–Au–Sb system at 870K, J. Alloys Compd., 2009, 479, 184–188 CrossRef CAS.
- Y. Chen, Y. Wei, P. Chang and L. Ye, Morphology-controlled synthesis of monodisperse silver spheres via a solvothermal method, J. Alloys Compd., 2011, 509, 5381–5387 CrossRef CAS.
- N. L. Tarwal, A. V. Rajgure, J. Y. Patil, M. S. Khandekar, S. S. Suryavanshi, P. S. Patil, M. G. Gang, J. H. Kim and J. H. Jang, A selective ethanol gas sensor based on spray-derived Ag–ZnO thin films, J. Mater. Sci., 2013, 48, 7274–7282 CrossRef CAS.
- Y. Wei, X. Wang, G. Yi, L. Zhou, J. Cao, G. Sun, Z. Chen, H. Bala and Z. Zhang, Hydrothermal synthesis of Ag modified ZnO nanorods and their enhanced ethanol-sensing properties, Mater. Sci. Semicond. Process., 2018, 75, 327–333 CrossRef CAS.
- J. Ding, J. Zhu, P. Yao, J. Li, H. Bi and X. Wang, Synthesis of ZnO–Ag hybrids and their gas-sensing performance toward ethanol, Ind. Eng. Chem. Res., 2015, 54, 8947–8953 CrossRef CAS.
- Q. Xiang, G. Meng, Y. Zhang, J. Xu, P. Xu, Q. Pan and W. Yu, Ag nanoparticle embedded-ZnO nanorods synthesized via a photochemical method and its gas-sensing properties, Sens. Actuators, B, 2010, 143, 635–640 CrossRef CAS.
- X. Xing, X. Xiao, L. Wang and Y. Wang, Highly sensitive formaldehyde gas sensor based on hierarchically porous Ag-loaded ZnO heterojunction nanocomposites, Sens. Actuators, B, 2017, 247, 797–806 CrossRef CAS.
- N. Hongsith, C. Viriyaworasakul, P. Mangkorntong, N. Mangkorntong and S. Choopun, Ethanol sensor based on ZnO and Au-doped ZnO nanowires, Ceram. Int., 2008, 34, 823–826 CrossRef CAS.
- L. Chengchao, L. Limiao, D. Zhifeng, Y. Hongchun, X. Yingying, L. Yuan, C. Yong and W. Taihong, Rapid and ultrahigh ethanol sensing based on Au-coated ZnO nanorods, Nanotechnology, 2008, 19, 035501 CrossRef PubMed.
- E. Wongrat, P. Pimpang and S. Choopun, Comparative study of ethanol sensor based on gold nanoparticles: ZnO nanostructure and gold: ZnO nanostructure, Appl. Surf. Sci., 2009, 256, 968–971 CrossRef CAS.
- J. Zhang, X. Liu, S. Wu, B. Cao and S. Zheng, One-pot synthesis of Au-supported ZnO nanoplates with enhanced gas sensor performance, Sens. Actuators, B, 2012, 169, 61–66 CrossRef CAS.
- T. Santhaveesuk, D. Wongratanaphisan and S. Choopun, Enhancement of sensor response by TiO2 mixing and Au coating on ZnO tetrapod sensor, Sens. Actuators, B, 2010, 147, 502–507 CrossRef CAS.
- Z. Yuan, X. Jiaqiang, X. Pengcheng, Z. Yongheng, C. Xuedong and Y. Weijun, Decoration of ZnO nanowires with Pt nanoparticles and their improved gas sensing and photocatalytic performance, Nanotechnology, 2010, 21, 285501 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta03224g |
| This journal is © The Royal Society of Chemistry 2020 |