A broadband aggregation-independent plasmonic absorber for highly efficient solar steam generation†
Abstract
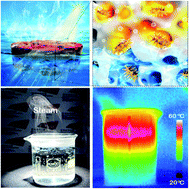
Achieving efficient solar steam generation under natural sunlight has huge potential for sewage purification and seawater desalination. Plasmonic resonance has been extensively exploited for enhancing and extending the range of optical absorption. Until now, most reported broadband plasmonic solar absorbers have been designed by compact aggregation or engineering plasmonic architectures. In this work, we develop a new plasmonic absorber using gold nanostructures with the shape of a trepang (nano-trepang). By rationally regulating anisotropy at the single nanoparticle level, the nano-trepang shows good optical absorption over the entire solar spectrum (92.9%) with no requirement of engineering nanoparticle aggregation or constructing plasmonic architectures. The nano-trepang was then loaded into a polymeric aerogel and the network showed an excellent solar-to-vapor energy conversion efficiency of 79.3%. Under 1 sun AM1.5 G irradiation, a stable solar evaporation rate of 2.7 kg m−2 h−1 can be achieved, with high performance anti-salt precipitation in practical seawater steam generation. This work shows a broadband plasmonic absorber with aggregation-independent performance for highly efficient solar stream generation and provides a new strategy for practical solar desalination.
- This article is part of the themed collection: Journal of Materials Chemistry A Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...