PAN/PI functional double-layer coating for dendrite-free lithium metal anodes†
Abstract
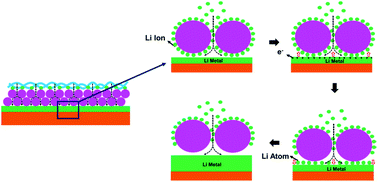
Li metal anodes attract intensive attention due to their high specific capacity, low density and the lowest electrochemical potential. However, inferior coulombic efficiency (CE) and safety hazards induced by uncontrollable Li dendrite growth are main obstacles against the commercialization of Li metal batteries (LMBs). Herein, we demonstrate that the polyacrylonitrile fiber/polyimide sphere (PAN fiber/PI sphere) double-layer coating can serve as an interfacial functional layer to guide uniform Li deposition and inhibit the formation of Li dendrites. Owing to polar groups and regularly arranged PI spheres of the coating layer, Li||PAN/PI@Cu cells display high coulombic efficiency and long cycle life at a current density of 1 mA cm−2, maintaining an average CE of ∼98.5% after 400 cycles with a deposition capacity of 1 mA h cm−2.


 Please wait while we load your content...
Please wait while we load your content...