Surface oxidized two-dimensional antimonene nanosheets for electrochemical ammonia synthesis under ambient conditions†
Abstract
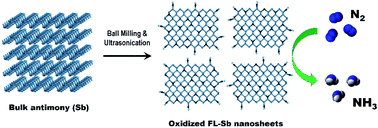
Two-dimensional (2D) antimonene nanosheets are prepared using a combination of ball milling and sonication-based liquid exfoliation and are used as an efficient electrocatalyst for the nitrogen reduction reaction (NRR). In 0.1 M KOH, a high NH3 yield of 180.4 μg h−1 mgCAT−1 and faradaic efficiency (FE) of 11.6% are achieved using our antimonene nanosheets. Theoretical calculations suggest that the oxidized species of antimonene act as the active catalytic sites for the NRR process. This work opens up a new avenue towards the development of 2D electrocatalysts for clean energy.
- This article is part of the themed collection: 2020 Journal of Materials Chemistry A most popular articles


 Please wait while we load your content...
Please wait while we load your content...