Neighboring effect induced by V and Cr doping in FeCoP nanoarrays for the hydrogen evolution reaction with Pt-like performance†
Abstract
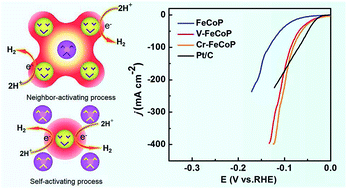
A ternary FeCoP nanoarray was used as a model catalyst to study the effect of doping 3d transition metals on the Hydrogen Evolution Reaction (HER) performance through density functional theory (DFT) and experiments. Two mechanisms were found to dominate this catalytic reaction: neighbor-activating and self-activating mechanisms. The electronegativity difference between the dopant and host elements was the main determinant of which mechanism the HER follows. It means that if the electronegativity of the doping element is smaller than that of Fe and Co, it would donate electrons to the neighboring host atoms which would become the active sites for the HER. Conversely, if the electronegativity of the doping element was larger than that of Fe and Co, it would attract charges from the neighbors, retarding the HER on host atoms. Based on the above findings, V- and Cr-doped FeCoP catalysts were confirmed to exhibit excellent HER performance (overpotentials of 32 and 28 mV at 10 mA cm−2, respectively) and remarkable catalytic stability quite close to that of the commercial Pt/C catalyst.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...