Rechargeable Zn-ion batteries with high power and energy densities: a two-electron reaction pathway in birnessite MnO2 cathode materials†
Abstract
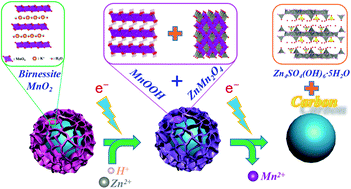
As one of the most promising next-generation safe and green energy storage technologies, aqueous Zn-ion batteries have attracted considerable attention in recent years. However, their working mechanism is still controversial. Also, their performance needs to be further improved to meet the requirement of widespread applications. Herein, we developed a nanoflower-like MnO2/C composite with two-electron reaction pathway for use as a cathode material in aqueous Zn-ion batteries. Benefiting from the large specific surface area of the nanoflower-like structure, high conductivity of the carbon materials and the low crystallinity of birnessite-type MnO2, the Zn-ion battery using MnO2/C as the cathode material could carry out a deep and fast reaction via the Mn4+/Mn2+ two-electron pathway. At 300 mA g−1, the battery could retain a high specific capacity of 279.7 mA h g−1 after 300 cycles. The second electronic reaction process of Mn3+/Mn2+ could also work at a high current density. A high initial discharge capacity of more than 200 mA h g−1 was achieved at a high current density of 2000 mA g−1. A two-electron working mechanism of the layered-MnO2 cathode material is proposed. Due to the advantages of high capacity, high rate performance and good stability, the MnO2/C composite is a promising cathode material for aqueous Zn-ion batteries, which holds potential for applications in electric vehicles and grid energy storage.


 Please wait while we load your content...
Please wait while we load your content...