Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalysis in Pickering emulsions†
Ana Maria Bago
Rodriguez
 * and
Bernard P.
Binks
* and
Bernard P.
Binks
 *
*
Department of Chemistry, University of Hull, Hull, HU6 7RX, UK. E-mail: b.p.binks@hull.ac.uk; a.bago-rodriguez@hull.ac.uk
First published on 13th November 2020
Abstract
Particle-stabilised or Pickering emulsions are versatile systems. In the past 10 years a new application has emerged in the field of catalysis to use them as vehicles to carry out catalytic reactions, allowing a more environmentally friendly process with high conversions and selectivities and important advantages for catalyst recovery. As the area has advanced rapidly, the intention of this review is to summarize the latest innovations being reported. An overview is given regarding the kinds of liquid phases comprising the emulsion system, the different types of solid particle stabilizers (whether they contain catalyst or not) and the catalytic reactions studied. A section describing methods for recovering the catalyst is also included, in which various stimuli are discussed. Finally, the importance of using Pickering emulsions to carry out reactions in flow and in multi-step cascade systems is highlighted with various examples to support the benefits of transferring this technology to industrial processes.
Professor Bernard P. Binks is Professor of Physical Chemistry at the University of Hull since 2003. He led the Surfactant and Colloid Group for 15 years with interests in surfactant phase behaviour, surfactant-stabilised foams and emulsions and wetting. Since 1998 his main research interest has been the behaviour of colloidal particles at fluid interfaces including oil–water, oil–oil, water–water, air–water and air–oil. This includes particle-stabilised emulsions, aqueous foams and oil foams. He has published 325 refereed papers, 16 patents and edited 3 research monographs. He was awarded the RSC Surfaces and Interfaces Award in 2014 and the ACS Langmuir Lectureship in 2016 and is currently a Senior Editor for Langmuir. |
1. Introduction
Catalysis is a well established and widely used strategy in the pharmaceutical and fine chemical industries.1 Over 75% of all chemicals are fully or partially obtained through a catalytic process and up to 90% of newly developed routes involve the use of catalysis.2 Despite the word catalysis being first introduced by Berzelius in 1835,3 the first definition based on physical chemistry principles was given in 1894 by Ostwald4 and it is still used nowadays. Catalysis is a term describing the process of increasing the rate of a chemical reaction by adding a small amount of a substance known as a catalyst, which is not consumed in the reaction and can be re-used several times as it undergoes no net chemical change. It lowers the activation energy of the reaction, allowing it to proceed through an alternative pathway to the one followed in the non-catalysed system and it can even affect the selectivity of chemical reactions. Therefore, catalysts are superior to stoichiometric reagents as their reaction efficiency minimizes the waste generated. That is why catalysis is considered one of the twelve principles of Green Chemistry.5Catalysts may be classified depending on whether they are in the same phase (homogeneous) or a different phase (heterogeneous) as/to the reaction medium. Homogeneous catalysts are molecularly dissolved in the reaction medium and all the atoms are active centers, which make them highly accessible to the substrate leading to high catalytic activity and selectivity even under mild conditions.6 However, once the reaction is completed, the separation of the catalyst from the reaction mixture is usually expensive and tedious and some catalysts can even cause serious corrosion problems to the equipment. Thus, despite their intrinsic advantages, homogeneous catalysts are used in less than 20% of industrially relevant processes.6 On the other hand, heterogeneous catalysts are easily separable and recyclable, which often results in lower operating costs.7 However, the stereoselectivity is still an issue and the active centers are restricted to the surface atoms. Moreover, mass transfer is limited because it requires the substrate to diffuse to the catalyst surface, adsorb, react and desorb back to the reaction medium. Therefore, a good catalyst must combine possession of well-defined active sites, high activity and selectivity of homogeneous catalysis with the insoluble solid characteristics of heterogeneous catalysis which allows easy separation and recyclability.
Despite the benefits catalysis can offer, a problem arises when the catalyst and the reactants are located in different and incompatible phases. Organic solvent–aqueous biphasic systems (unmixed) often suffer from low reaction efficiency due to their high mass and heat transfer resistance, which is linked to the limited organic solvent–aqueous interface across which the reaction can take place. However, Regen and the group of Ikeda reported an increase in catalytic activity in reactions carried out at a liquid (organic solvent)–liquid (water) planar interface without stirring when the surface of the catalyst particles was modified in such a way that allowed their adsorption at the interface.8–11 In ref. 9 an epoxidation reaction at the phase boundary between aqueous hydrogen peroxide and an alkene was evaluated. The catalyst, sodium zeolite impregnated with titania, is completely hydrophilic. By surface modification with octadecyltrichlorosilane (OTS), particles with intermediate and high hydrophobicity could be prepared.9 The highest catalytic activity was measured in the system containing particles of intermediate hydrophobicity, as they were adsorbed strongly at the liquid–liquid interface, unlike completely hydrophilic or completely hydrophobic particles which remained dispersed either in the aqueous or the organic phase, respectively.9 However, in general for the reaction to occur in two-phase systems, vigorous mechanical agitation must be implemented. By doing so, one of the liquids is homogenized into droplets and dispersed in the other liquid under continuous high-speed agitation to form an emulsion. However, droplets are large in size and prone to coalescence once agitation is halted. This continuous agitation and the direct contact between the aqueous and organic phases can be detrimental to enzyme activity in biocatalytic reactions. Consequently, fine control on the conversion, selectivity and yield is difficult to attain. To overcome this, co-solvents or phase-transfer reagents are employed but require the introduction of extra additives or involved procedures to modify catalysts.12,13 The addition of surfactants, followed by homogenisation, increases the interfacial area and facilitates transfer between phases. However, surfactants can be difficult to separate from final product mixtures and they can be harmful to the environment.14
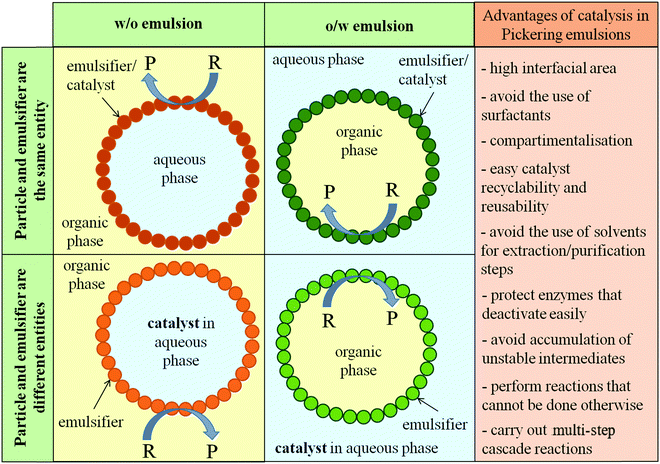
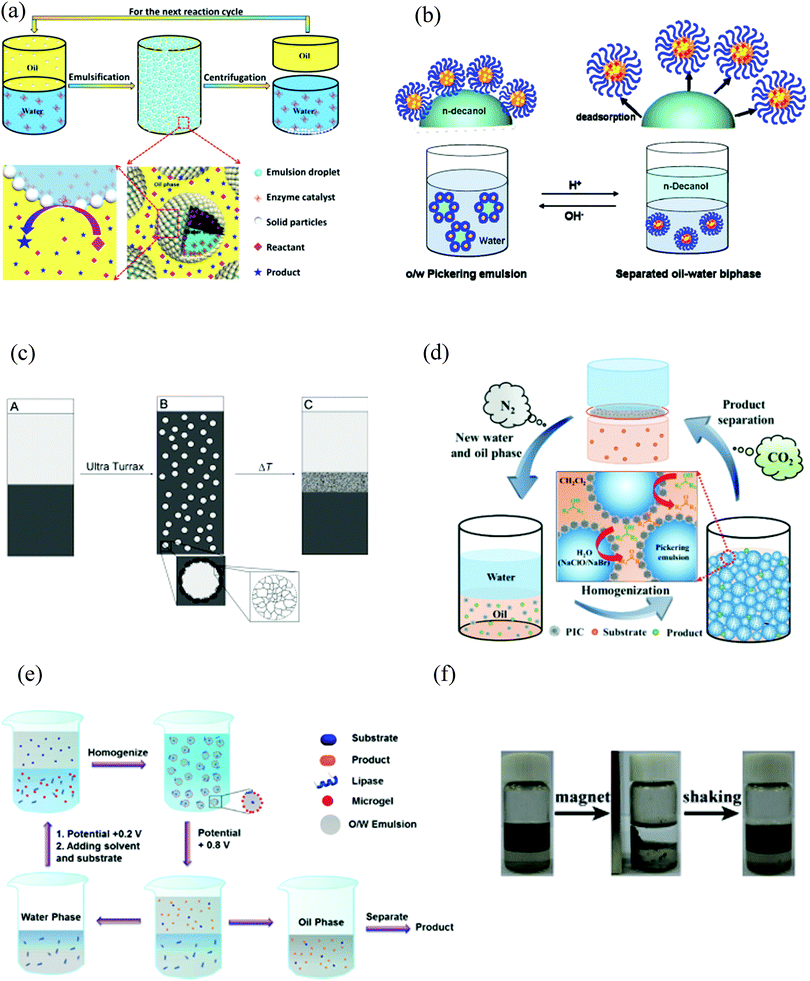
Emulsions stabilized by solid particles, so-called Pickering emulsions, have brought special attention in the past 10 years to the catalysis area due to their facile separation and recycling, among other advantages summarized in Fig. 1. As in emulsions stabilized by surface-active molecules (e.g. surfactant or polymer) one of the phases is divided into micrometer-sized droplets dispersed within the other incompatible liquid, which dramatically increases the interfacial area available for chemical reaction compared to typical two-phase systems. Therefore, agitation is unnecessary as each droplet acts as a micro-compartment that enhances the encounter of catalyst and reactant.15,16 Notably, solid particles, unlike surfactant molecules, may be irreversibly anchored at the liquid–liquid interface due to their high energy of detachment,17,18 and in the majority of cases these particles are biocompatible and environmentally friendly.
The catalyst in Pickering emulsions can either be dispersed/dissolved in one of the phases or be integrated within the solid particle stabilizer. Pera-Titus et al. advanced the phrase Pickering Assisted Catalysis (PAC) when the catalyst and the particle stabilizer are separate entities or Pickering Interfacial Catalysis (PIC) when the stabilizer and the catalyst form the same unit.19 The first example of catalysis in Pickering emulsions where the particles are both the emulsifier and the catalyst was given by Crossley et al.20 The hydrodeoxygenation of a phenolic compound and hydrogenation and etherification of an aldehyde were studied in water-in-oil (w/o) Pickering emulsions stabilized with Pd/carbon nanotube-inorganic oxide hybrid nanoparticles.20 Subsequently, approximately 100 or so publications have followed as described throughout this review.
The influence of the parameters normally evaluated in Pickering emulsion characterization, such as the average droplet diameter, oil-to-water ratio and particle concentration and wettability, have been added to those typically assessed in catalytic reactions: reaction conversion, efficiency, yield and selectivity. The main methods to tune the average droplet diameter in Pickering emulsions are the stirring/homogenization speed and the amount of emulsifier. At high speed and/or high particle content the average droplet diameter decreases, increasing the interfacial area. Wei et al. reported an increase of the specific activity of Candida Antarctica lipase B (CALB) by increasing the particle concentration in the hydrolysis kinetic resolution of racemic esters in w/o Pickering emulsions stabilized with partially hydrophobic silica nanospheres (Fig. 2(a)).15 By increasing the emulsifier content, not only does the droplet diameter decrease but the diffusion distance of the reactant molecules is reduced. However, at relatively high particle concentration, the activity almost reached a plateau. By increasing the amount of emulsifier, the coverage of droplet surfaces by particles increases, which compromised the reaction efficiency as the encounter between the substrate and the enzyme was reduced.15 Zhang et al. studied the hydrogenation of benzene to cyclohexene for different average droplet sizes in oil-in-water (o/w) Pickering emulsions prepared at different stirring rates.21 As shown in Fig. 2(b), an increase of the benzene conversion and the cyclohexene yield and selectivity were measured upon decreasing the average droplet diameter. Jiang et al. prepared w/o Pickering emulsions stabilized by silica particles of various sizes in the nanometer range to follow the esterification reaction between 1-hexanol and hexanoic acid catalyzed by lipase from Candida sp.22 As shown in Fig. 2(c) the conversion increased by decreasing the particle size due to a decrease in the average droplet diameter. The specific activity of the enzyme in the Pickering emulsion stabilized with 50 nm silica particles was 21 times higher than that of the free enzyme in a biphasic system without stirring.22 Li et al. studied the effect of the particle size (90–460 nm) and surface roughness of catalytic nanoparticles on the reactivity of a non-aqueous immiscible mixture (ethyleneglycol/dodecanal) in Pickering emulsions. The catalytic productivity was enhanced for smaller sized and rough nanoparticles (<210 nm) as a result of the formation of more compact interfacial particle assemblies and a higher specific surface area of the particles, reducing diffusion resistances near the acid sites.23 Finally, regarding the effect of the oil-to-water ratio, Yu et al. studied this on the catalytic efficiency for the hydrolysis of olive oil in o/w Pickering emulsions.24 A higher efficiency of the enzymatic transformation was achieved upon decreasing the volume fraction of oil in the emulsion due to a reduction in the droplet size. However, to fully understand the effect of the volume fraction of drops, the droplet size should be kept constant. Wang et al. carried out the enzymatic esterification of hexanoic acid with 1-hexanol in an o/w high internal phase emulsion (HIPE) with a large volume fraction of oil.25
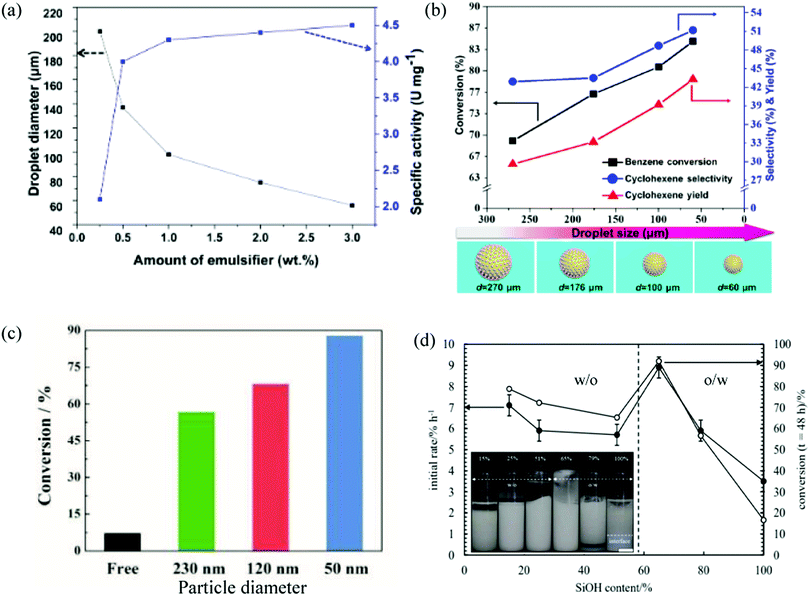 | ||
| Fig. 2 Influence of different formulation parameters on catalysis in Pickering emulsions. (a) Specific activity of Candida Antarctica lipase B and droplet diameter versus the amount of emulsifier (partially hydrophobic silica nanospheres modified with methyltrimethoxysilane) in the hydrolysis of (R,S)-1-phenylethyl acetate in w/o Pickering emulsions. Reproduced from ref. 15 with permission from the American Chemical Society. (b) Benzene conversion, cyclohexene selectivity and cyclohexene yield in the selective hydrogenation of benzene to cyclohexene versus average droplet size in o/w Pickering emulsions formulated at different stirring rates and stabilized with Ru/titania nanoparticles modified with (MeO)3SiCH3. Reproduced from ref. 21 with permission from Wiley. (c) Conversion after 20 min in the esterification of 1-hexanol with hexanoic acid catalyzed either by free lipase dispersed in water in a biphasic system or free lipase within the dispersed phase of w/o emulsions stabilized with silica nanoparticles of different size. Reproduced from ref. 22 with permission from Wiley. (d) Plot of the initial rate for the enzymatic dehydration of n-octanaloxime to n-octanenitrile catalysed by an aldoxime dehydratase (OxdB) overexpressed in E. coli and conversion of the reaction versus silica particle hydrophobicity (given by SiOH content) used to prepare either w/o or o/w emulsions (emulsion type is given in inset). Reproduced from ref. 29 with permission from Wiley. | ||
The stabilization mechanism within Pickering emulsions is based on the strategic location of solid particles at the interface and such effect is explained by the partial particle wettability and the contact angle that particles exhibit at the oil–water interface.26,27 For instance, hydrophilic silica particles contain surface hydroxyl groups and by reaction with dichlorodimethylsilane (DCDMS) the silanol (SiOH) content can be reduced to render increasingly hydrophobic silica particles. Emulsions of different types, stabilities and droplet diameters can be prepared with silica particles of different wettability.28 Bago Rodriguez et al. show for the first time the effect of particle hydrophobicity on a dehydration reaction catalysed by an aldoxime dehydratase (OxdB) overexpressed in E. coli.29 When comparing two emulsions of different type with similar stability and droplet diameter, the o/w system (prepared with 65% SiOH silica particles) displayed a higher conversion than the w/o system (prepared with 51% SiOH silica particles) (Fig. 2(d)).
The use of Pickering emulsions as vehicles to carry out catalytic reactions, despite only being studied in the past decade, has witnessed significant progress and important advantages have been reported not only regarding their potential to increase the conversion and selectivity of reactions,30 but also to develop more environmentally friendly processes which are in high demand. This concept arises from the combination of various branches within pure science, such as physical chemistry, inorganic chemistry, organic chemistry and biochemistry, which reveals the multi-disciplinary character and the wide range of potential applications. Pera-Titus et al. published a mini review in 2015, dedicating two sections to describe some of the first examples of PAC and PIC.19 The area, however, has advanced rapidly and requires a comprehensive review summarizing the latest technologies and the different strategies being used. In this review, we first give an overview of the different aspects to consider when designing a reaction in Pickering emulsions, such as the liquid phases involved, particle stabilizer (whether they contain the catalyst or not) and the catalytic reaction itself. We also dedicate a section dealing with methods to recover the catalyst other than centrifugation. Finally, we introduce the concept of using Pickering emulsions to carry out reactions in flow and in multi-step cascade systems, which paves the way to implement this technology in industrial processes.
2. Liquids used in Pickering emulsions for catalysis
Various liquids have been used as the two phases to carry out catalytic reactions in Pickering emulsions. These include a wide range of oils (non-polar alkanes, aromatic hydrocarbons and vegetable oils), polar solvents (water, ethylene glycol, ethyl acetate) and ionic liquids (IL). These solvents give rise to the preparation of many types of emulsions (o/w, w/o, IL/o and w/w), as summarized in Table S1 (ESI†) following an extensive review of the literature. In this collection of references, the catalytic reaction as well as details of the particles and catalyst are included and will be described in the following sections.Water-in-water systems, also known as aqueous two-phase systems (ATPSs) or all-aqueous systems are formed upon phase separation of an aqueous mixture of two incompatible polymers (usually polyethylene glycol (PEG) and dextran) or a polymer and a salt.31,32 Despite the advantages ATPSs could bring to the field of biocatalysis compared to typical water–oil emulsions, these systems are not extensively used as the long-term stabilisation is still difficult to attain due to the ultralow water–water interfacial tension (typically 1 μN m−1)33 and the intrinsic thick interface.34 Certain colloidal particles, however, are promising emulsifiers for ATPSs. Chao and Shum include in their review (Table 2) various examples of w/w systems stabilised by solid particles.35 Despite these challenges, various catalytic reactions in w/w emulsions can be found in the literature.35–42 Lipid vesicle-stabilised w/w emulsions have been utilized for the production of mineral calcium carbonate by local enzymatic production of carbonate ions with urease in dextran-rich aqueous droplets dispersed in a continuous PEG-rich aqueous phase.39 The same ATPS was stabilised by PEGylated liposomes by Dewey et al. to study a ribozyme cleavage reaction using a two-piece hammerhead ribozyme, demonstrating their potential as microscale bioreactors.38 Cakmak and Keating studied the distribution of three natural clay mineral particles (kaolinite, montmorillonite and illite) in dextran-in-PEG ATPSs and their ability to catalyse the reaction of o-phenylenediamine with peroxide to form 2,3-diaminophenazone.37 Finally, Xue et al. prepared a methoxy PEG-urease conjugate to use both as a stabiliser of w/w emulsions of dextran and PEG and as the interfacial biocatalyst for the hydrolysis of urea to ammonium carbonate.36
Although water is a recognized green solvent, it still has many limitations such as low solubility for organic substrates, low tolerance for water-sensitive compounds/reactions and a low boiling point.43 As an alternative to the classical green solvents, ILs have emerged as potential candidates in Pickering emulsion systems for catalysis due to the following reasons: (i) they are environmentally benign non-aqueous solvents with high thermal and chemical stability and low saturated vapor pressure,44 (ii) they have good solvent properties towards both ionic and covalent compounds and enzymes, (iii) their tunable structure and properties enables them to be used in a wide range of reactions and (iv) they are immiscible with some non-polar solvents which allows the formation of emulsion droplets upon homogenisation. ILs have been identified as efficient catalysts for the cyanosilylation of carbonic compounds.45 Moreover, they can replace the harmful dichloroethane often used as the reaction medium in this transformation. One drawback of ILs however is their relatively high viscosity.44 Therefore, if this reaction is conducted in batch, mechanical agitation is not particularly efficient and can hamper the production at large scale.45 However, in Pickering emulsions, bulk IL is broken into numerous droplets on the micrometer scale which increases the interfacial area and benefits fast diffusion of reactants and products without the need for agitation. As mentioned above, enzymes can be dissolved in ILs, and this phase can be used to prepare IL/o Pickering emulsions. Various biocatalytic reactions were studied in ref. 46 and 47. The use of IL as the dispersed phase is crucial as it confines the biocatalyst and allows the exchange of molecular reactants with the continuous organic phase.47 ILs can even in some cases enhance the selectivity of a reaction.43,45,46,48 Tao et al. carried out the hydroformylation of long chain alkenes in an IL/o Pickering emulsion system.48 The overall catalytic performance was superior to that of a w/o system. Despite both emulsion types exhibiting similar conversions, the IL/o emulsion displayed higher chemoselectivity.48 The unique properties of ILs enabled the reaction to go through hydroformylation pathways for enhancing chemoselectivity to aldehydes. Moreover, differences in the recycling stability were observed between the two emulsion types. While for the w/o Pickering emulsion system the conversion and aldehyde selectivity decreased on the third cycle, no obvious decrease within the first six cycles could be observed in the IL/o Pickering emulsion system.48
The conversion within a chemical reaction could vary depending on the emulsion type, i.e. which liquid phase is dispersed in which. Bago Rodriguez et al. have recently investigated the effect of the conversion of n-octanaloxime to n-octanenitrile in Pickering emulsions stabilized by silica particles of different hydrophobicity.29 This allowed comparing the conversion of the reaction in two emulsions of different type with the same particle type, average droplet diameter and stability to coalescence and creaming/sedimentation. The conversion in that case was higher in the o/w system.29 Although this is the first systematic study of the effect of particle wettability on the conversion of a reaction, other attempts were previously carried out. Meng et al. prepared mesoporous silica particles of different wettabilities by using silylating agents with different carbon chain lengths.49 However, only w/o emulsions could be prepared with substantial fractions of water and oil released in some cases.49 Therefore, the catalytic evaluation was only carried out in emulsions of one type, with the conversion higher for the emulsion with the highest stability against sedimentation and coalescence and smaller droplets.49 In ref. 50, Pd supported on amphiphilic carbonaceous microspheres (Pd/CM) was used in the catalytic hydrodeoxygenation of vanillin as a model bio-oil upgrading reaction. The wettability of the Pd/CM catalyst could be easily tuned by adjusting the hydrothermal treatment temperature during synthesis or by a post-treatment process in alkaline solution.50 However, emulsions could only be prepared with particles of intermediate hydrophobicity, with other particles being either too hydrophilic or too hydrophobic. The emulsion type was, instead, strongly dependent on the oil volume fraction.50 Thus w/o or o/w emulsions were prepared when the oil-to-water ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The effectiveness of the Pickering emulsion for the reaction was tested in both emulsion types, displaying a similar conversion but different selectivities of p-cresol, being 60% and 38% after 1 h in the w/o and o/w emulsion, respectively.50
2, respectively. The effectiveness of the Pickering emulsion for the reaction was tested in both emulsion types, displaying a similar conversion but different selectivities of p-cresol, being 60% and 38% after 1 h in the w/o and o/w emulsion, respectively.50
Examples of non-aqueous mixtures are also being reported.23,51–53 Here, the liquids are both the phases comprising the emulsion and the reactants. Xu et al. studied an acetalization reaction between dodecyl aldehyde and ethylene glycol in emulsions stabilized with carbon-based acid catalysts.51 Similarly, the esterification reaction of glycerol with dodecanol is evaluated in simple and double emulsion systems stabilized with polystyrene-grafted silica nanoparticles bearing sulfonic acid centers.52 Finally, Yang et al. used modified silica particles to stabilize a methanol-in-vegetable oil Pickering emulsion for a transesterification reaction.53
3. Pickering emulsion stabilizers and their role in the catalytic reaction
Pickering emulsion stabilizers frequently attach irreversibly at the liquid–liquid interface of dispersed droplets.17,18 During emulsification the interfacial area increases dramatically, allowing the reaction to occur faster compared to the quiescent planar interface. As mentioned in the introduction, for reactions carried out in Pickering emulsions, the catalyst and emulsifier can either be separate entities (PAC) or a single particle can have dual functionality (PIC).(a) Pickering assisted catalysis
In general, examples of PAC are widely found in the field of biocatalysis, where the enzyme (catalyst) is dissolved in the aqueous phase and the solid particles are located at the liquid–liquid interface.15,22,24,47,54–57 In most of these examples, silica particles were selected as the emulsifier for the preparation of w/o emulsions to study hydrolysis and esterification reactions using lipase enzymes as the biocatalyst dissolves in water. However, in ref. 24, an o/w emulsion is prepared with the enzyme dispersed in the continuous phase and in ref. 47 the biocatalyst is found in the IL phase constituting an IL/o emulsion. Silica particles have been widely employed as stabilizers in Pickering emulsions due to their high biocompatibility, stability and easy surface modification. These characteristics make them also suitable for catalysis.Some examples where the enzyme is part of the emulsifier, i.e. particles have dual functionality, are also found in the biocatalysis area. One example of this kind is the use of polymersomes – self-assembled vesicles from amphiphilic block copolymers – which can encapsulate enzymes in their lumen (polymer bilayer). This enables the aggregates to act both as the stabilizer and the catalyst for the esterification of 1-hexanol and hexanoic acid in w/o Pickering emulsions (Fig. 3(a)).58 Other examples are based on the use of mesoporous silica particles or zeolites (aluminosilicates).22,48,49,59–63 They are widely employed as enzyme carriers due to their porous nature, which brings a large internal surface area where enzymes can be adsorbed and protected from the organic solvent. Moreover, due to their composition, they have outstanding biocompatibility and thermal and mechanical stability.49,59 Despite silica nanospheres being broadly employed as a stabilizer in Pickering emulsions, the influence of the particle meso-structure on the catalytic performance of Pickering emulsions has rarely been investigated. Zhao et al. compared the catalytic activity in the hydroformylation of 1-octene with water-soluble Rh-tris(m-sulfonatophenyl) phosphine complexes as catalysts of two o/w Pickering emulsion systems stabilized either by mesoporous or non-porous silica nanospheres with a similar particle diameter (100 nm).60 The emulsion with non-porous silica particles displayed a much lower turnover frequency (TOF) and aldehyde selectivity than that prepared with mesoporous silica particles.60 While the reaction mainly takes place at the oil–water interface and the diffusion of reactants and products occurs only through the voids between adsorbed silica nanoparticles, with mesoporous silica the reaction could take place both at the interface and in the nanopores.60 Moreover, the mesopores provide an additional diffusion channel for reactants and products. Another example of immobilization of enzymes on porous silica particles is demonstrated by Wang et al., where CALB is immobilized on silica nanoflowers modified with DCDMS to produce biodiesel from waste oil, as a biodegradable, non-toxic and renewable alternative.62 Silica nanoflowers (0.5 μm in diameter) are a kind of porous silica material with center-radial pore structure. The high accessibility to guests and large internal surface area coming from the open pore superstructure can facilitate the loading of enzyme molecules and the mass transfer of substrates.64 The maximum biodiesel yield was 98.8% when the reaction was carried out in a w/o Pickering emulsion stabilized with CALB immobilized in the porous material, which was nearly four times higher than that with free CALB.62 Moreover, after repeated use for 15 times, the yield of biodiesel catalyzed by the Pickering emulsion system was 77% while that obtained with free CALB was virtually zero.62 This indicates that immobilization could effectively improve the stability of the enzyme, enabling the use of enzymes as re-usable and robust biocatalysts.61 Meng et al. immobilized lipase in mesoporous silica particles modified with alkyl silanes with a diameter between 350–450 nm and radially arrayed pores of about 2–5 nm (Fig. 3(b)).49 The transformation of tributyrin to butyric acid was evaluated in water-in-paraffin oil Pickering emulsions and was catalyzed by Lipase B from Pseudomonas cepacia (PCL). While the starting material was dispersed in the oil phase, the final product was recovered in the water phase. The encapsulation efficiency of the enzyme in the mesoporous particles is about 78% and the main driving forces for enzyme adsorption inside pores are hydrophobic interactions, electrostatic attraction, hydrogen bonding and van der Waals forces.49 An example of the use of zeolites (Fig. 3(c)) for biofuel upgrading reactions is given by Zapata et al.63
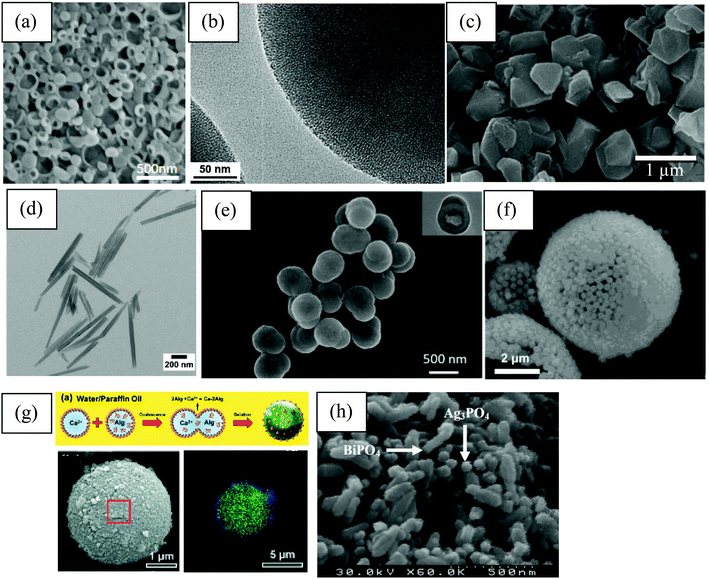 | ||
| Fig. 3 Examples of different kinds of particles used in catalysis in Pickering emulsions. (a) SEM image of cross-linked polymersomes. The enzyme CALB is dispersed either in the aqueous phase or within the polymersome lumen to investigate the esterification of 1-hexanol and hexanoic acid in w/o Pickering emulsions (reproduced from ref. 58 with permission from Wiley). (b) TEM image showing the porous nature of mesoporous silica particles. Lipase from Pseudomonas cepacia will be adsorbed inside the pores to catalyse the conversion of tributyrin to butyric acid in w/o Pickering emulsions (reproduced from ref. 49 with permission from Multidisciplinary Digital Publishing Institute). (c) High resolution transmission electron microscopy of octadecyltrichlorosilane-functionalized zeolite used as the emulsifier and the catalyst for biofuel upgrading reactions (reproduced from ref. 63 with permission from the American Chemical Society). (d) TEM image of hydrophilic Halloysite (natural clay of molecular formula Al2Si2O5(OH)4·nH2O) nanotubes used as emulsifier. The catalytic centre (Rh-sulfonated 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene) is dispersed in the water phase of an o/w Pickering emulsion to evaluate the hydroformylation of olefins (reproduced from ref. 68 with permission from the American Chemical Society). (e) SEM and TEM (inset) images of poly(1-vinyl-3-ethylimidazolium bromide) (PIL) functionalized silica@polystyrene/polyvinylbenzene Janus particles (JP). The catalyst H3PW12O40 was then immobilized on PIL-JP by anion-exchange to catalyse acylation reactions (reproduced from ref. 43 with permission from Elsevier). (f) SEM image of TEOS-treated micron sized Pickering w/o emulsion droplets stabilized by silica nanoparticles. Lipase from Candida sp. is encapsulated within droplets to study the esterification between 1-hexanol and hexanoic acid (reproduced from ref. 22 with permission from Wiley). (g) (Top) Schematic representation of the preparation of lipase-immobilised surface-active alginate microparticles through alginate gelation via the coalescence of two w/o Pickering emulsions decorated with silane-grafted TiO2 nanoparticles to perform an esterification reaction. (Bottom left) SEM and (bottom right) confocal laser scanning microscopy images of an alginate microparticle. Green and blue indicate FITC-labelled lipase and silane-grafted TiO2 nanoparticles, respectively (reproduced from ref. 90 with permission from The Royal Society of Chemistry). (h) SEM images of Ag3PO4/BiPO4 composites used as the catalyst and as the emulsifier (in combination with multi-walled carbon nanotubes) in photocatalytic reactions (reproduced from ref. 83 with permission from The Royal Society of Chemistry). | ||
Apart from enzymes, other types of catalysts have been used for PAC, such as acids or bases,55,65–67 metallic catalysts48,68 and even ionic liquids.45 Yang et al. use HCl and NaBH4 in w/o Pickering emulsions stabilized with partially hydrophobic silica nanospheres for a deacetalization-reduction cascade reaction.66 Silica particles are also used as the particulate emulsifier by Xue and co-workers, where H2SO4 and ethylenediamine are the catalysts for the deacetalization-Knovenagel condensation cascade reaction in w/o Pickering emulsions.67 The cyclization of citronellal using H3PW12O40 (an heteropolyacid) as a catalyst and silica particles as the stabilizer of a w/o emulsion has also been reported.65 Regarding the use of metallic catalysts, Stehl et al. used silica nanoparticles and Halloysite nanotubes (Fig. 3(d)) to stabilize o/w Pickering emulsions as a vehicle to carry out the hydroformylation of a long chain olefin.68 Rhodium was used as the catalyst dissolved in the aqueous continuous phase by the ligand sulfonated 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene.68
(b) Pickering interfacial catalysis
Examples of PIC, other than those in the field of biocatalysis with porous silica, MgO or polymersomes, have also been extensively reported.20,51–53,69–80 In the vast majority of the systems, the catalytic activity is attributed to Pd, which is supported on various surface-active particles such as silica or titania amongst others.16,20,71,72,75,77,78 In ref. 72, Pd nanoparticles supported on silane-modified graphene oxide were used as the Pickering emulsion stabiliser and efficient catalyst for the selective hydrogenation of cinnamaldehyde. Zhang et al. studied the palladium-catalysed nitroarene reduction with NaBH4 as a model reaction to evaluate the reaction efficiency in three systems under static conditions: w/o Pickering emulsion, phase-boundary reaction (with the catalyst at the interface of the biphasic system) and a biphasic reaction (with the catalyst dispersed in the water phase).16 The former gave the highest conversion (97%), followed by the phase-boundary system (12%) and then the biphasic reaction (<1%). The surface-active catalyst was prepared by depositing palladium nanoparticles onto triamine-octyl-bifunctionalized silica microspheres.16 A wide variety of materials such as silica, iron oxide or bentonite clay are often used to stabilise emulsions. However, most of them require surface modification to make them interfacially active. Han and co-workers prepared surface-active Pd/C catalyst by loading Pd nanoparticles on carbon spheres as support for the hydrogenation of a series of aromatic compounds.77 Carbon materials, which possess hydrophilic groups (e.g. carboxyl and hydroxyl) at the edges and hydrophobic polyaromatic benzene rings within the basal plane, might assemble at the interface of water and oil to form a Pickering emulsion without further modification.77Metals other than Pd have also been immobilised in/on particles for interfacial catalysis. Zhang et al. used Ru deposited on titania particles modified with (MeO)3SiCH3 for the selective hydrogenation of benzene in o/w and w/o emulsions.21 In ref. 81, cellulose nanofibers with aldehyde groups were chosen as the solid emulsifier to stabilize Pickering emulsion droplets, coated with a series of metal nanocatalysts (Pd, Au, Pt) via a facile aldehyde-induced reduction method to study the reduction of various organic substrates by NaBH4.
Cakmak and Keating employed three natural clay mineral particles (kaolinite, montmorillonite and illite) both as stabilisers for a dextran-in-PEG ATPS and as catalysts for the reaction of o-phenylenediamine with peroxide to form 2,3-diaminophenazone.37 While montmorillonite was not surface-active, both illite and kaolinite could stabilise droplets but illite was more active in this reaction.37
Mineral acids, such as H2SO4, H3PO4 and HF, are used as homogenous acid catalysts for several applications despite their corrosive nature and the difficulty in recycling at the end of the chemical process.51 If used, they require a neutralisation step, which makes the process more polluting and expensive. Therefore, in order to implement more environmentally friendly processes with easier methods for product recovery, these mineral acids must be replaced by solid acids.82 Solid particles containing polysulfonic acid groups as catalysts at the particle surface have been reported.23,51–53,70,73 Yang et al. functionalised silica nanoparticles with alkyl chains and polysulfonic acid residues to provide the particles with both surface activity and catalytic properties.53 The usefulness and effectiveness of emulsions stabilised by such particles as reaction vehicles was evaluated for the catalytic transesterification of vegetable oils using methanol under mild conditions.53 Xu et al. functionalised activated charcoal with phenylsulfonic groups and used these particles to stabilise dodecylaldehyde-in-ethylene glycol Pickering emulsions for a solvent-free acetalization reaction.51 Despite both silica and carbonaceous materials being used for the synthesis of heterogeneous acid catalysts and regardless of the fact that silica materials are easier to be functionalized than carbonaceous materials, the Si–O bond breaks easier in basic or acidic media than the C–C bond. However, the same reaction has been studied in Pickering emulsions with silica particles functionalised with polysulfonic acid groups by Zhou and co-workers.70
(c) Characteristics of the particle stabiliser
In general, spherical particles are used to stabilize emulsions although other shapes have been reported, including nanotubes (Fig. 3(d)),30,74,83,84 nanofibers,81 fractal fumed silica and graphene oxide sheets.72 Very recently, Zhang et al. used titanate nanotubes modified with CH3Si(OCH3)3 as the particle stabilizer and titanate nanotubes containing Ru nanoparticles in the interior of the hollow tubular structure as the catalysts to study the selective hydrogenation of α,β-unsaturated aldehydes.30 It was found that the assembly of catalyst particles precisely at the inner interfacial layer of w/o Pickering emulsion droplets gave a much higher selectivity than those at the outer interfacial layer, within the interior of droplets and at the conventionally-called Pickering emulsion interfaces (including both outer and inner layers).30 This study shows the importance of the precise control of the location of catalyst particles on catalytic behavior.Cellulose nanofibers can entangle together to form a robust layer to encapsulate emulsion droplets. Moreover, their large surface area allows the deposition of large amounts of catalyst.81 Janus particles are a special type of particle whose surfaces have two or more distinct physical properties (Fig. 3(e)).85 This feature has proved advantageous in the field of catalysis.43,86–88 Faria et al. used Janus particles to stabilize o/w Pickering emulsions using decalin as the organic phase.86 The Janus particles, themselves synthesized via a Pickering emulsion strategy, consist of hydrophilic silica particles functionalized on one side with aminopropyltriethoxysilane rendering it hydrophobic. Before emulsification, the surface of the particles was modified by anchoring Pd on them, either on the entire surface or on the hydrophobic side only. This allowed investigating the concept of phase selectivity in emulsions by following the hydrogenation of two aldehydes with different solubilities: benzaldehyde (oil-soluble) and glutaraldehyde (water-soluble).86 When the catalytic Janus particles contained Pd on both sides, high conversions were measured for both reactants. However, when the catalyst was selectively deposited on the hydrophobic side, the conversion of glutaraldehyde was only 2%.86
Sometimes the presence of the particle stabilizer at the liquid–liquid interface is not sufficient to bring the desired robustness for a specific application.46,55,67 Cross-linking of interfacial particles forming an inorganic shell around the droplets may be required, e.g. for flow applications to avoid leakage of the internal liquid46 or to assemble droplets containing opposing reagents in the same vessel for multi-step cascade reactions.67 Recently, Bago Rodriguez and Binks reviewed methods to form capsules from Pickering emulsion templates including the sol–gel process.89Fig. 3(f) shows the appearance of a TEOS-treated micron sized w/o emulsion droplet stabilized by silica nanoparticles. Yang et al. on the contrary prepared surface-active soft particles for emulsion stabilization via ionic gelation from the coalescence of two w/o emulsions, one containing calcium chloride and the other containing sodium alginate (Fig. 3(g)).90
Yet another way of stabilising Pickering emulsions is with mixtures of particles, especially those of opposite charge.91–93 Inspired from this idea, Yang et al. used the colloidal tectonic approach to prepare emulsions stabilized from the interaction between cationic [C12]3[PW12O40] nanoparticles (resulting from the ionic attraction between dodecyltrimethylammonium chains and phopshotungstate anions) and anionic silica nanoparticles grafted with alkyl and sulfonic acid groups.73 The self-assembly of both nanoparticles is driven by partial penetration of the alkyl chains of one into the other.73 Another example of catalysis in Pickering emulsions stabilized with self-assembled nanohybrids (Ag3PO4/BiPO4 with multi-walled carbon nanotubes/graphene) at the liquid–liquid interface is given by Mohaghegh et al. (Fig. 3(h)).83
Finally, the hardness of the particle stabiliser can also have an impact on the catalytic process. While typical Pickering emulsion stabilizers are hard particles (mainly from inorganic sources such as silica), some emulsions have been stabilized with soft particles like microgels or from polyelectrolyte complexes.94–97 Only a few examples of catalysis in Pickering emulsions stabilized by microgel/nanogel particles or a mixture of microgels (soft) and solid particles (hard) are reported.98–101 Microgels are soft cross-linked polymer particles that are able to adsorb at liquid–liquid interfaces and swell or contract under the action of an external stimulus.102,103 Microgels might provide further advantages for biocatalysis because it is known that enzyme immobilization within can enhance enzyme stability and thus recyclability.100 The adsorption of the microgel to the oil–water interface will locate the enzyme in close proximity to the oil phase, while still preventing direct contact. However, the shear conditions in the preparation of the emulsion have to be moderate to avoid enzyme deactivation, the emulsion must be stable at the temperature where the enzyme is active and the breaking of the emulsion with an external stimulus must avoid enzyme denaturation. Wiese et al. investigated three different types of microgels (one negatively charged and two uncharged) as stimuli-sensitive emulsion stabilisers for enzyme-catalysed reduction of acetophenone to (R)-phenylethanol.99 Despite the reaction yield being independent of the type of microgel, the conditions for emulsion breaking differed. Recently, Huang et al. prepared a food-grade o/w Pickering emulsion system for biphasic biocatalysis.101 Here, a lipase was electrostatically adsorbed onto the surface of a chitosan nanogel (∼50 nm), providing the particles with both emulsifying and catalytic properties to study the hydrolysis of p-nitrophenyl palmitate.101
4. Types of catalytic reaction studied in Pickering emulsions
The type of catalytic reaction will be governed by the type of catalyst. Various reactions have been studied, including examples in the field of photocatalysis, biocatalysis, chemocatalysis, acid or base catalysis or IL-catalysed reactions.(a) Acid-catalysed reactions
As mentioned in the previous section, grafting active polysulfonic acid residues to surface-active nanoparticles brings them catalytic properties and allows an easy recovery of the catalyst. Yang et al. studied the acid-catalyzed transesterificartion of vegetable oils using methanol under mild conditions in methanol-in-vegetable oil Pickering emulsions stabilized with silica particles with grafted alkyl chains and polysulfonic acid residues (Cn–SiO2–SO3H) (Fig. 4(a)).53 This consists of three sequential reactions (triglyceride → diglyceride → monoglyceride → glycerol) where one fatty acid methyl ester molecule is removed in each step. The degree of conversion depends on the length of the alkyl chain grafted on the particles (n), being greater with n = 18. The time required to obtain 98% conversion of sunflower oil is 12 h, while the conversion values measured after the same period of time for the control experiments with H2SO4 were substantially lower.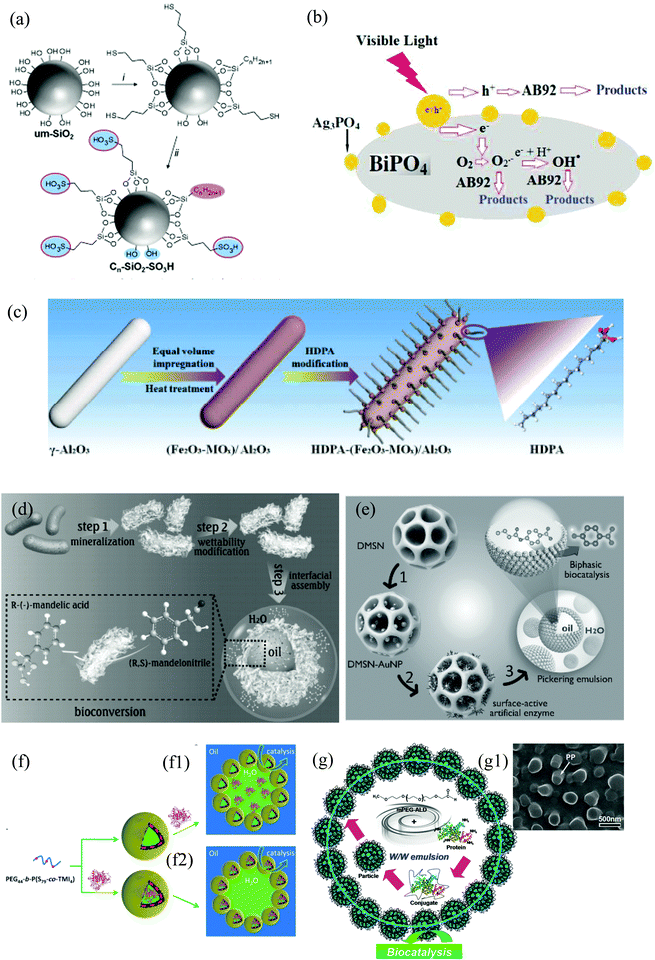 | ||
| Fig. 4 Schematic illustrations for the preparation of various catalysts used in a wide range of reactions in Pickering emulsions. (a) Acidic/amphiphilic silica nanoparticles for an acid-catalyzed reaction. The hydrophobic (alkyl chain), hydrophilic (alcohol) and catalytic (sulfonic acid) domains are represented as a red shade, blue shade and blue/red circle, respectively. Reproduced from ref. 53 with permission from The Royal Society of Chemisty. (b) Possible mechanism for AB92 azo dye degradation by the Ag3PO4/BiPO4 heterojunction photocatalyst under visible light irradiation. Reproduced from ref. 83 with permission from The Royal Society of Chemistry. (c) Hexadecylphosphate acid (HDPA)-terminated mixed-oxide nanoparticles for an oxidation reaction. Nano γ-Al2O3 is used as support for doped mixed-oxides (Fe2O3–MOx; where M = Mn, Co, Ni, Cu, Cr, Mo, V, Ti), loaded via a wet impregnation method. The resulting entities are then decorated with HDPA. Reproduced from ref. 104 with permission from Elsevier. (d) Encapsulated bacteria, used both as the emulsifier and the catalyst, for a biotransformation (hydrolysis of hydrophobic (R,S)-mandelonitrile to hydrophilic R-(−)-mandelic acid) is prepared as follows: (i) deposition of a calcium phosphate mineral shell doped with Fe3O4 nanoparticles onto the bacterial surface (mineralization), (ii) adsorption of sodium monododecyl phosphate (wettability modification). Reproduced from ref. 109 with permission from Wiley. (e) Surface-active artificial enzymes used as interfacial biocatalysts are obtained by: (i) synthesis of dendrimer-like mesoporous silica nanoparticles (DMSN) with octyl group-modified outer surface and aminosilane and thiolsilane co-modified inner surface. Gold nanoparticles (AuNPs) are deposited on the inner surfaces of DMSN, (ii) deposition of thiol-ended catalytic groups on the AuNPs to form the catalytic loci. Three kinds of catalytic groups (peptides, CeIV-nitrilotriacetic acid complex or G-quadruplex/hemin) are employed to construct surface-active particles (with esterase-, phosphotriesterase- and peroxidase-like activities, respectively). Reproduced from ref. 110 with permission from Wiley. (f) Polymersome building block poly(ethylene glycol)-b-poly(styrene-co-3-isopropenyl-α,α-dimethylbenzylisocyanate) (PEG-b-P(S-co-TMI)) is prepared by reversible addition–fragmentation chain-transfer polymerization of styrene and TMI employing a PEG44 chain-transfer agent. (f1) and (f2) represent a w/o Pickering emulsion droplet stabilized with polymersomes with the enzyme either in the water phase (f1) or in the polymersome lumen (f2) for enzymatic catalysis. Reproduced from ref. 58 with permission from Wiley. (g) Design of polymer–protein conjugate particles with biocatalytic activity for the stabilization of water-in-water emulsions. Methoxy PEG (mPEG)-Bovine Serum Albumin (BSA) conjugates are prepared by reacting mPEG-acetaldehyde (ALD) with BSA at a pH near the protein isoelectric point. (g1) SEM image of mPEG-BSA conjugate particles, PP. Reproduced from ref. 36 with permission from the American Chemical Society. | ||
(b) Photocatalytic reactions
Photocatalytic reactions are important from an environmental viewpoint as the process can easily be activated by absorption of light. Fessi et al. used titania photocatalysis as a promising green alternative for water treatment.76 1-Methylnaphthalene(1-MN)-in-water Pickering emulsions stabilized by catalytic titania nanoparticles were prepared. Through light-induced redox reactions at the surface of the photocatalyst, highly oxidizing active oxygen species were formed which allowed the degradation of organic substrates.76 For comparison, the Pickering emulsion system and a non-emulsified 1-MN–water mixture in the presence of titania particles were irradiated. While the overall concentration of 1-MN progressively decreased during the photodegradation process in both systems, the reaction was significantly faster in the emulsified system, where 95% could be degraded after 24 h of UV irradiation, compared with only 25% in the non-emulsified system. The larger oil–water interfacial area in the former maximized the contact between the photocatalyst and the pollutant which intensified the reaction.76 Wang et al. also took advantage of the photocatalytic properties of titania to study the production of hydrogen in o/w Pickering emulsions of bio-octanol and water.87 Finally, a different example of the use of Pickering emulsions in photocatalysis is that given by Mohaghegh et al.83 In this system, a novel p–n heterojunction Ag3PO4/BiPO4 (AB) acts as the photocatalytic active component while hydrophobic multi-walled carbon nanotubes (MWCNT) or graphene (GR) confer surface activity and also have the ability of capturing and shuttling electrons through the π–π network and hence allow electron transfer as shown in Fig. 4(b). The photocatalytic activity was studied by monitoring the change in the Acid Blue 92 dye concentration under visible and UV light irradiation, being higher with the AB/GR or AB/MWCNT Pickering emulsion system than with the traditional solution system.83(c) Oxidation reactions
Deng et al. evaluated the oxidation of toluene to benzaldehyde by liquid oxygen, a reaction highly demanded by industry in the past decades as the main route has serious environmental and corrosion problems and the benzaldehyde produced contains a small amount of halogens.104 Liquid oxidation of toluene by oxygen with noble metal-based catalysts including Pd, Au, Pt, Ag and Ru has become popular owing to their excellent catalytic activity. Deng and co-workers report the doping effect of a series of metal oxides (MO) on the catalyst hexadecylphosphate acid (HDPA)–FeOx (Fig. 4(c)) using γ-Al2O3 as a support. The (Fe2O3–MOx)/Al2O3 samples were prepared by an incipient wet impregnation method. Afterwards HDPA was loaded on the catalyst.104 The conversion of toluene is ∼71% and benzaldehyde is the only product formed when HDPA-25Fe2O3/Al2O3 is used as catalyst. A control experiment with 25Fe2O3/Al2O3 as catalyst reports a conversion of toluene below 8% with a lower selectivity of benzaldehyde (∼40%). The by-products formed are benzyl alcohol (20.3%) and benzoic acid (39.6%). This implies that the modification with HDPA greatly promotes the toluene conversion and ensures an exclusive selectivity in this catalytic system.(d) Nucleophilic substitutions
Yang et al. carried out a nucleophilic substitution to produce iodooctane from the reaction of bromooctane with NaI in an o/w Pickering emulsion stabilised with graphene oxide grafted with polyethylene glycol and 3-aminopropyltriethoxysilane.105 Despite an increase in the amount of nucleophilic reagent (NaI) being beneficial to the reaction, a high concentration of salt was detrimental to the emulsion stability and hence decreased the conversion of bromooctane.(e) Enzymatic reactions
Enzymes are powerful biocatalysts and display, even with complex substrates, high chemo-, regio- and stereo-selectivities under mild conditions.106 Therefore, they could be potential replacements to the non-renewable fossil resources traditionally used in chemical industries, which again fulfils one of the goals of green chemistry. In a chemical reaction, however, organic substrates have generally poor solubility in the water phase where enzymes are typically active. Therefore, to perform biocatalysis in Pickering emulsions, it is crucial to bring the enzymes to the liquid–liquid interface to allow contact with substrates but at the same time they have to be protected from the organic solvent as it can be detrimental to the enzyme activity. As introduced in Section 3, various methods to fulfil these two vital requirements have been extensively reported in the literature and can be divided between those where the enzyme is dispersed in the aqueous phase and protected from the organic environment by a shell of solid particles at the liquid–liquid interface or those where enzyme-immobilized particles are anchored at the droplet interface. The latter can be accomplished by confinement in hollow particles or mesoporous silica particles,49,59,61,62 conjugation to polymer107 or to metal–organic frameworks108 or encapsulation in gels/microgels90,100 amongst others. Moreover, the enzyme-immobilisation method maximizes the contact area between enzyme and substrate and reduces the diffusion distance of substrate molecules. However, the process of enzyme immobilization involves solvent-exchange, temperature alteration or chemical bonding, which could significantly affect the activity, selectivity and stability of the enzyme. As a result, microgels are a good option.100Chen et al. described the encapsulation of individual living cells within robust artificial shells as a Pickering stabilizer for the hydrolysis of hydrophobic (R,S)-mandelonitrile to hydrophilic R-(−)-mandelic acid in emulsions.109 As shown in Fig. 4(d)Alcaligenes faecalis ATCC 8750 cells were first coated with a porous calcium phosphate mineral shell and Fe3O4 nanoparticles were doped simultaneously into the shell to endow bacteria with magnetic functionality (B-MCaP). Sodium monododecyl phosphate was adsorbed on the mineral shell, to render B-MCaP interfacially active (B-MCaPS). For comparison, control experiments with a collection of bacterial cells located in the droplet interior of silica particle-stabilized w/o Pickering emulsions and a collection of bacterial cells immobilised in calcium alginate beads were conducted. In the Pickering emulsions stabilized with B-MCaPS, the conversion of the reaction reached 90–95% at equilibrium, whereas for the silica-stabilised Pickering emulsion equilibrium took longer to be reached.109 Although in both cases the interfacial area increases and so does the mass transfer, the slower conversion in the latter case was due to internal diffusional limitations within the droplets. Finally, only about 40% of conversion after 24 h was detected when bacteria were immobilized in calcium alginate beads. This low catalytic efficiency could again be linked to external and internal diffusional limitations.109
Chen et al. also encapsulated enzymes in mesoporous silica nanoparticles for interfacial biocatalysis.110Fig. 4(e) shows the different steps for their complex synthesis. Firstly, dendrimer-like mesoporous silica nanoparticles (DMSN) with octyl group-modified outer surfaces and aminosilane and thiolsilane co-modified inner surfaces were prepared. Gold nanoparticles (AuNPs) were deposited on the inner surfaces of DMSN. Afterwards, thiol-ended catalytic groups were deposited on the AuNPs to form the catalytic loci. Three kinds of catalytic groups (peptides, CeIV-nitrilotriacetic acid complex or G-quadruplex/hemin) were employed to construct surface-active particles (with esterase-, phosphotriesterase- and peroxidase-like activities, respectively).110 The assembly of the particles at o/w and w/o emulsion droplet interfaces dramatically enhanced the catalytic activity due to the greatly enlarged oil–water interfacial area and favoured entry/egress of substrates and products. Moreover, these particles exhibited significantly higher operational stability against long-term exposure to organic solvents, as highlighted by the impressive re-usability for more than 20 cycles and high storage stability over 30 days with negligible loss of catalytic activity.110
As described earlier, polymersomes are self-assembled vesicles from amphiphilic block copolymers with a size range of 200–500 nm and can be considered a special type of hollow colloidal nanoparticle for enzyme loading.111 Moreover, if they display surface activity they can be used for enzymatic transformations in Pickering emulsions. Wang et al. compared the catalytic performance of an enzyme in the esterification of 1-hexanol and hexanoic acid in two w/o emulsion systems stabilized with polymersomes.58 In the first approach CALB was dissolved in the water phase, while in the second case the enzyme was loaded in the polymersome lumen followed by emulsification (Fig. 4(f)). The conversion of the reaction reached 80–90% in both systems, while less than 25% conversion after 24 h was measured in the biphasic system. When CALB was encapsulated in the lumen of the polymersome, the specific activity was nearly three times higher than that when positioned in the water phase of the Pickering emulsion.58 Xue et al. carried out a similar comparison of the catalytic activity for the enzymatic hydrolysis of urea to ammonium carbonate in w/w emulsions stabilised with mPEG-urease conjugate particles (Fig. 4(g)).36 Higher catalytic activity was obtained when the enzyme was incorporated in the particulate emulsifier compared to when it was dispersed in the dextran-rich droplets stabilised by denatured mPEG-urease in a PEG-rich continuous phase.36
5. Recyclability and re-usability of catalysts
In general, catalysts are expensive and not readily abundant. Hence, once the reaction is completed the catalyst must be easily recovered and the activity must be maintained to ensure high efficiency in subsequent reaction cycles.The most straightforward and basic method to separate the catalyst from the mixture is to centrifuge the emulsion in order to separate the two liquid phases, followed by isolating the catalyst from one of the phases though filtration (Fig. 5(a)). In homogeneous catalysis or when the catalyst particle size is in the submicrometer-to-micrometer range, filtration is not the best separation technique as the filter pores have to be small enough to trap the catalyst. Moreover, catalysts can remain attached to the filter, being difficult to recover. The process of centrifugation and filtration is time and energy consuming and not easy to adapt to the industrial scale where large volumes are handled. Therefore, demulsifying the emulsion with less aggressive methods is essential if we are to see Pickering emulsions flourish as vehicles to carry out catalytic reactions in industrial processes. An alternative method to centrifugation would entail the adsorption/desorption on demand of the particle stabilizer from the liquid–liquid interface using an external stimulus, such as pH,69,75,100,112,113 temperature,99 CO224,114 or electrochemical98 or magnetic responses.109,112 These particles designed with specific features can stabilize emulsions which can be broken on demand. However, some of them might not be suitable for specific catalytic systems. For instance, pH-responsive systems will cause the accumulation of salts which may be harmful to ionic strength-sensitive systems. In ref. 113, the high salt build up did not allow the reaction to be carried out more than 6 cycles as the polymer precipitates from the water phase. However, Xi et al. demonstrated that an emulsion prepared with sodium caseinate (forming micelles) as the sole emulsifier could be recycled over 100 times as its pH-responsive property was maintained even in a saturated salt solution (NaCl ∼6.1 M).115 Thermo-responsive systems on the other hand are energy demanding and if used in the field of biocatalysis, the enzyme has to be stable at the temperature where the stabilization/destabilization occurs.98 Described below are examples of various methods used to recover the catalyst other than centrifugation.
 | ||
| Fig. 5 Schematic illustration of various methods to demulsify (and recover) Pickering emulsions. (a) Centrifugation of emulsions prepared with partially hydrophobic silica nanospheres modified with methyltrimethoxysilane (reproduced from ref. 15 with permission from the American Chemical Society). (b) pH-responsive Pickering emulsion stabilized with Au@poly(ethylene oxide)-b-poly(4-vinylpyridine) (Au@PEO-b-P4VP) polymer–metal hybrid micelles. Demulsification at low pH is caused by the high swelling of the protonated P4VP cores, which enhance the hydrophilicity and drive the hybrid micelles to desorb from the oil–water interface. Upon increasing the pH, re-adsorption at the interface takes place (reproduced from ref. 113 with permission from Springer). (c) Temperature-responsive Pickering emulsion stabilized with one of the following microgels: poly(N-isopropylacrylamide-co-methacrylic acid) (negatively charged), poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) (uncharged) or poly(N-isopropylacrylamide)-poly(N-isopropylmethacrylamide) (uncharged core–shell). The emulsion is broken by increasing the temperature above the VPTT of the microgels (reproduced from ref. 99 with permission from Wiley). (d) CO2/N2-responsive Pickering emulsion stabilized with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) immobilised in P((TMA-co-DMA)-b-MMA). Bubbling with CO2 causes demulsification due to the polymer deprotonation (reproduced from ref. 114 with permission from The Royal Society of Chemistry). (e) Potential-responsive Pickering emulsions stabilized with 8-arm PEG-cyclodextrin and 8-arm PEG-ferrocene self-assembled into potential stimulated microgel particles. The electrochemical response allows the reversible formation and deformation of microgels which causes the assembly and disassembly from the oil–water interface (reproduced from ref. 98 with permission from the American Chemical Society). (f) Magnetic-responsive Pickering emulsion stabilized with Alcaligenes faecalis cells coated with porous calcium phosphate mineral shell and Fe3O4 nanoparticles. Sodium monododecyl phosphate was adsorbed afterwards to turn the encapsulated bacteria interfacially active. Complete phase separation occurred after approaching a strong magnet and once the magnet was removed, emulsions could be formed again after homogenisation (reproduced from ref. 109 with permission from Wiley). | ||
(a) pH-Responsive
A pH-responsive o/w Pickering emulsion stabilized by polymer–metal hybrid micelles (Au@poly(ethylene oxide)-b-(poly(4-vinylpyridine))) has been used as a catalytic microreactor for the reduction of p-nitroanisole with NaBH4.113 At low pH, the protonated poly(4-vinylpyridine) cores swell and the enhanced hydrophilicity drives the hybrid micelles to desorb from the oil–water interface into the water phase. Upon increasing the pH, the hybrid micelles re-adsorb at the interface as shown schematically in Fig. 5(b). Another interesting application where the pH change plays an important role is that given by Yang et al.75 Silica microspheres were modified with hydrophobic (MeO)3Si(CH2)7CH3 and pH-sensitive (MeO)3SiCH2CH2CH2(NHCH2CH2)2NH2 and Pd was deposited afterwards. These particles had a double function as stabilizer and catalyst for the hydrogenation of styrene. Firstly, the pH was adjusted to 3–4 and an o/w emulsion was prepared. Once the reaction is completed, in order to separate the product from the catalyst, the pH value was re-adjusted to 7–8, leading to emulsion inversion to w/o with some extra oil resolved. Hence, the solid catalyst is transferred into the bottom layer and a neat oil phase is resolved, which can simply be removed by decantation.75 The solid catalyst can be transferred back into the upper layer after adjusting the pH value for the next reaction cycle. This protocol allowed the system to be recycled for 36 times.75(b) Temperature-responsive
“Smart” microgel particles that respond to temperature variations changing their size, softness and hydrophobicity have been evaluated as stimuli-responsive emulsifiers for carrying out a bioenzymatic transformation.99 After emulsification, surface-active microgels are located at the droplet surface and the reaction, catalyzed by an enzyme within the aqueous droplets, takes place. Once the reaction is completed, the w/o emulsion is destabilised by increasing the temperature above the volume phase transition temperature (VPTT) of the microgel. The organic phase containing the reaction product can then be easily separated. On the other hand, the aqueous phase contains the biocatalyst and the flocculated microgel, which can be re-dispersed in the aqueous phase upon cooling and re-used for another catalytic cycle after adding fresh organic phase with substrate.99 This process is schematically represented in Fig. 5(c). Yet another interesting example of a stimulus-responsive system is that given by Huang et al. where the wettability of a chitosan nanogel (stabiliser) was modified in situ by interaction of the nanogel with free fatty acids triggered by lipase hydrolysis. This enzymatic trigger allows rapid and controlled release of active components.101(c) CO2-Responsive
Among the stimuli, CO2 has attracted considerable interest for being green, pollution-free, cheap and highly biocompatible.114 Tang et al. immobilised 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) in amphiphilic block polymers (P((TMA-co-DMA)-b-MMA) or P(TMA-b-MMA)) and used one of these Pickering interfacial catalysts in a typical biphasic reaction to estimate the catalytic activity of alcohol oxidation under stirring and non-stirring conditions.114 The surface wettability of the polymeric interfacial catalysts was evaluated via contact angle measurements with water, showing a decrease in the contact angle by increasing the amount of relatively hydrophilic 2-(dimethylamino)ethyl methacrylate, DMA. As a result, either an o/w or w/o Pickering emulsion could be prepared. The dichloromethane-water system was chosen as a model to evaluate the CO2-responsive behaviour of the PIC. CO2 was bubbled into the emulsions stabilised with either of the two catalysts once the reaction was completed to trigger fast demulsification (Fig. 5(d)). As a control, P(TMA-b-MMA) without DMA cannot demulsify under CO2 bubbling. Subsequently, the PIC was deprotonated by bubbling N2 and the PIC could be directly used for a new reaction system. The conversion of benzyl alcohol catalysed with P((TMA-co-DMA)-b-MMA-3) was 99% after 5 cycles.114(d) Electrochemical-responsive
Electrochemical stimuli can also be used to break emulsions on demand as shown in ref. 98 (Fig. 5(e)). As electrochemical-responsive Pickering emulsions are non-accumulative and can be stimulated at room temperature, these systems are highly desirable in the field of biocatalysis. Ferrocene (Fc) functionalized 8-arm poly(ethylene glycol) star polymer, (8A PEG-Fc) is able to self-assemble into potential stimulated microgels.98 Fc is oxidized at a potential of +0.8 V and reduced at +0.2 V. This electrochemical response allows the reversible formation and deformation of the microgels. An o/w Pickering emulsion was prepared, containing Lipase from Pseudomonas cepacia (PCL) in the aqueous phase to catalyze the hydrolysis of triacetin and the kinetic resolution of (R,S)-1-phenylethanol.98 The formation and deformation of the microgel can be regulated by an external potential, thus endowing the Pickering emulsion with controlled reversibility. The oxidation of Fc induces the phase separation. The product is soluble in the organic phase, thus can be easily separated, while the enzyme remains in the water phase and is ready for the next reaction cycle after adding fresh organic phase containing substrate (Fig. 5(e)).(e) Magnetic field-responsive
Finally, an example of a Pickering emulsion responsive to a magnetic field is shown in ref. 109 (Fig. 5(f)). Alcaligenes faecalis ATCC 8750 cells were firstly coated with a porous calcium phosphate mineral shell, followed by the addition of Fe3O4 nanoparticles to endow bacteria with magnetic functionality (B-MCaP). After adsorption of sodium monododecyl phosphate on the mineral shell, the hydrophilic B-MCaP became interfacially active (B-MCaPS) facilitating the stabilization of both o/w and w/o Pickering emulsions.109 By modifying the mineral-shell surfaces with increasing concentration of sodium monododecyl phosphate (MDP) o/w emulsions were transformed into w/o emulsions. With a strong magnetic field, complete phase separation was observed and when the magnet was removed, emulsions could be re-formed after homogenization. This could facilitate the in situ recycling of the biocatalyst and enable the separation of the products without the need of an energy source or addition of chemicals. The hydrolysis of mandelonitrile was carried out using these modified cells as Pickering interfacial biocatalyst and conversion efficiencies as high as 80% were obtained after 30 cycles.1096. Pickering emulsions in flow
A known volume of Pickering emulsion can be poured into a column reactor at the bottom of which is a membrane/filter that prevent droplets from leaking but which allows the continuous phase of the emulsion with the substrate/reactant to percolate due to gravity or by applying pressure (Fig. 6(a)). Therefore in these flow systems, reactants dissolved in the same solvent as the continuous phase of the emulsion are continuously fed from the inlet and products continuously collected at the outlet. Due to this constant renewal of the continuous phase, higher conversions and selectivities in Pickering emulsions and conventional biphasic systems have been reported compared with batch reactions. This can also in some cases be beneficial to avoid the product inhibition effect. By increasing the flow rate, the conversion decreases and the catalytic efficiency increases. Higher conversions are obtained by decreasing the flow rate because of larger retention times for the reactant in the column reactor. In terms of large-scale application, the flow reaction system is superior to the batch reaction counterpart since the products do not need to be separated from the emulsion after each batch, which is cost-inefficient and time- and energy-consuming.81 By varying the average droplet diameter in w/o emulsions, the flow rate changes. Zhang et al. reported a decrease in the oil flow rate by decreasing the water droplet size.55 This can be explained by the fact that at fixed volume fraction of water there is a larger number of water drops when they are small compared to when they are large. As oil has to flow around more drops when they are small, this reduces the flow rate. Flow Pickering emulsion systems usually encapsulate catalysts in the droplets, so care should be taken in continuous flow processes as the continuous phase may extract the catalysts to some degree and consequently affect the catalytic performance.43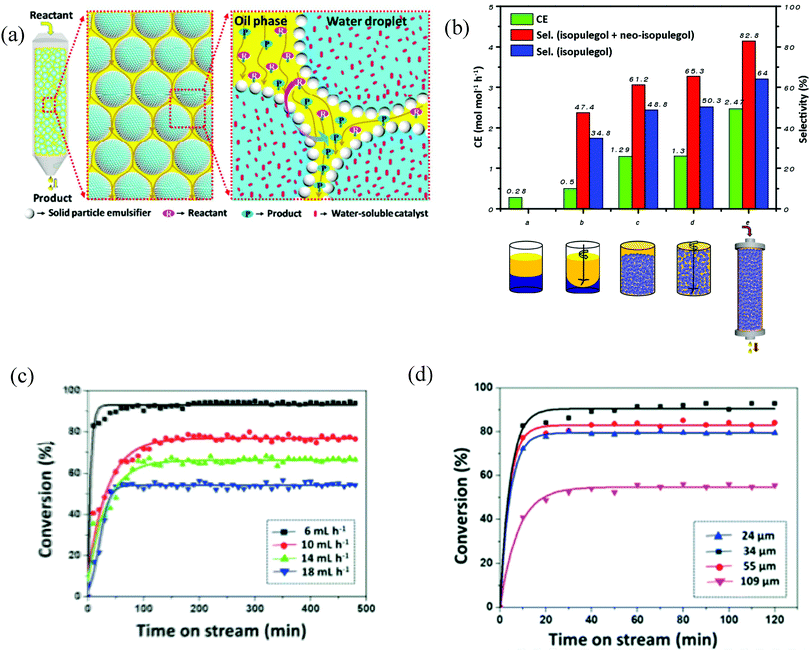 | ||
| Fig. 6 Pickering emulsions in flow. (a) Schematic representation of the flow Pickering emulsion strategy for oil–water biphasic catalytic reactions (reproduced from ref. 55 with permission from the American Chemical Society). (b) Catalysis efficiency (CE) and selectivity (Sel.) for isopulegol + neo-isopulegol and isopulegol (two diastereoisomers generated from the cyclization of citronellal) in different catalytic systems: (a) stir-free conventional biphasic system, (b) conventional biphasic system stirred at 900 rpm, (c) stir-free w/o Pickering emulsion, (d) w/o Pickering emulsion stirred at 900 rpm and (e) flow w/o Pickering emulsion (reproduced from ref. 65 with permission from Wiley). (c and d) Conversions with time for acetophenone cyanosilylation in flow IL/o Pickering emulsions at different flow rates (c) and droplet sizes (d) (reproduced from ref. 45 with permission from The Royal Society of Chemistry). | ||
In ref. 55 a w/o emulsion was first prepared with hydrophilic silica nanospheres modified with methyltrimethoxysilane and then the emulsion was packed in a column (Fig. 6(a)). The stability of compartmentalized water droplets was maintained by measuring the flow rate with time which remained constant. Moreover, no detectable water was found in the collected eluents even upon application of high flow rates. Various catalytic reactions were carried out in this set up with high conversions and the values were maintained for various days: H2SO4-catalysed addition reaction, HPA-catalyzed ring opening reactions and an enzyme catalyzed reaction to promote the kinetic resolution of racemic esters to chiral alcohol.55 For the first reaction, further experiments were carried out to compare the catalysis efficiency measured in the biphasic system (agitation-free and stirred), a Pickering emulsion system (with and without agitation) and the flow Pickering emulsion system. The agitation-free biphasic reaction gave a catalytic efficiency (CE) of 0.0013 mol mol−1 h−1. This value improved substantially up to 0.0106 mol mol−1 h−1 when agitated. For the static and stirred Pickering emulsion systems, the CE was further increased up to 0.023 mol mol−1 h−1 and 0.024 mol mol−1 h−1, respectively. The sufficiently large reaction interface area makes agitation unnecessary for reactions in Pickering emulsions. Finally, with the flow reaction in Pickering emulsions the CE was of 0.235 mol mol−1 h−1, which is about 20 times higher than that of the conventional batch stirred biphasic reaction system and 10 times higher than the batch Pickering emulsion reaction.
Chen et al. developed a continuous-flow catalysis system for the cyclization of citronellal in w/o emulsions.65 H3PW12O40 (HPA) was chosen as the water-soluble catalyst and partially hydrophobic silica nanospheres with diameters of 40–60 nm were used as the solid emulsifier. To evaluate this reaction, the flow Pickering emulsion system was compared with batch systems including conventional biphasic reactions and Pickering emulsion reactions (Fig. 6(b)). The stir-free conventional biphasic system only transformed <20% citronellal within 20 h while stirring could dramatically improve the catalysis efficiency up to 0.5 mol mol−1 h−1 due to the enhanced mass transport. However, the activity of HPA catalyst was still limited since only 60% conversion was obtained after 24 h. In the Pickering emulsion system, the cyclization proceeded faster and the conversion reached to above 93% after 15 h. Finally, the flow Pickering emulsion showed not only the best catalytic efficiency of 2.47 mol mol−1 h−1 (nearly 5 times higher than that of the conventional biphasic system and 2 times higher than that of the Pickering emulsion batch reaction) but a dramatic selectivity enhancement.65 The influence of the droplet size on the conversion in flow Pickering emulsions was studied by changing the amount of solid emulsifier. Decreasing the droplet size results in an increase in the total water–oil interface area but at the same time leads to an increase in the droplet surface coverage, C.65 This means that two or even more particle layers form on the droplet interfaces, which can compromise the contact of the reactants with the catalyst, thereby reducing the catalytic efficiency. More interestingly, the stereoselectivity also changed with the droplet size. To further confirm this flow interface catalysis effect, the authors examined the effect of the flow rate on the conversion. By decreasing the flow rate the retention time for the reactants was longer, which led to a higher conversion and lower catalysis efficiency.65
Another example regarding the effect of the droplet size on the flow rate is reported by Zhang et al. in an IL/o flow Pickering emulsion. Under the same applied pressure, larger droplets result in a higher flow rate of the oil phase.47 Tang and co-workers carried out a Friedel–Crafts reaction in a continuous flow IL/o Pickering emulsion.43 Again, the flow Pickering emulsion showed a relatively higher conversion than that obtained with batch systems of the stir-free and stirred IL/o Pickering emulsion. In order to explore the effect of emulsion droplet size in the reaction, droplet sizes were tuned by changing the amount of IL. The conversion increased with decreasing the emulsion droplet size which led to larger interfacial areas.43
A further example of the use of IL is that given by Meng et al.45 A continuous-flow cyanosilylation reaction system is developed based on the confinement of one catalytically active IL catalyst dissolved within the other IL droplets in an IL/o Pickering emulsion. The conventional batch reactor gave a catalytic efficiency of 9.1 mol mol−1 h−1. In contrast, in the continuous flow system it was as high as 21.4 mol mol−1 h−1. The impact of the oil flow rate on the catalytic efficiency was investigated by conducting the continuous-flow reaction at different flow rates (Fig. 6(c)). As the flow rate increases, the acetophenone conversion decreases but the catalytic efficiency increases. Reactant molecules around catalytically active sites are greatly increased when raising the flow rate, and accordingly more reactant available can be converted into product.45 The impact of droplet size on the catalytic efficiency was then evaluated by varying the amount of emulsifier as shown in Fig. 6(d). By increasing the amount of emulsifier, the average droplet diameter decreases and the catalytic efficiency first increases and then decreases, revealing an optimal droplet size range for obtaining high catalytic efficiency.45 The reactant molecules initially dissolved in n-octane can enter the IL droplets through diffusion, due to the relatively large partition coefficient of the reactant in the IL. For the reaction within the IL droplets, there are two factors that govern the observed reaction rate: molecular diffusion rate and catalytic reaction rate. The authors assume that there is a critical reaction depth (distance from the droplet surface), which reflects the molecular diffusion distance. When the droplet radius R is exactly equal to the critical reaction depth, the time scale of molecular diffusion amounts to the time scale of the catalyzed reaction.45 This means that catalysts within the droplet are fully utilized and the reactant molecules are just completely converted into product. When R is greater than the critical value, the reactant molecules need longer time to diffuse to the droplet center and accordingly the catalytic efficiency is lower. Conversely, when R is less than the critical value, there are insufficient catalyst molecules in the droplets to react with the incoming substrate molecules and the excess substrate molecules must flow out from the droplets.
7. Pickering emulsions for multi-step cascade reactions
A potential important application of Pickering emulsions is to use them as a vehicle to carry out multiple consecutive reactions.20,51,53,66,67,73,107,116 This has the advantage that it avoids purification and isolation of intermediates saving time, energy and resources.66 Moreover, a Pickering emulsion system enables one to carry out reactions with incompatible reagents by separating the opposing reactants in different compartments.66 This procedure consists of preparing two parent w/o Pickering emulsions each containing one of the conflicting reagents. Afterwards, these emulsions are mixed together by stirring67 or via a lamination procedure,66 which consists of forming alternating layers of the two emulsions. Yang et al. carried out a deacetalization-reduction cascade reaction in Pickering emulsions stabilized with partially hydrophobic silica nanospheres, where HCl and NaBH4 catalyse the deacetalization and reduction reactions, respectively.66 In principle, it is impossible to combine these two reactions in a single vessel as HCl and NaBH4 rapidly react with each other releasing hydrogen. Using the layered Pickering emulsion strategy, after 30 min benzaldehyde dimethylacetal was fully transformed to the final product benzyl alcohol. As the layer thickness decreases, the content of benzyl alcohol and the reaction rate increases. Moreover, the content of the intermediate benzaldehyde was always lower than 5% and the accumulation of the intermediate did not occur during the whole course of the reaction.66 The versatility and effectiveness of the Pickering emulsion strategy was further demonstrated with other cascade reactions including different pairs of opposing reagents: deacetalization-Knovenagel condensation cascade (acid/base pair), deacetalization-Henry cascade (acid/base pair) and diazotization-iodization cascade (oxidant/reductant pair). Of special interest is the last reaction as in the conventional process there is a serious risk of explosion due to the accumulation of unstable diazonium in the reaction system. This does not occur here as when formed it is immediately transformed into iodides. As shown in Fig. 7(a), this Pickering emulsion strategy was also used in a one-pot four cascade reaction for the synthesis of mono N-alkyl amine using nitrobenzene and benzaldehyde dimethylacetal as starting materials.66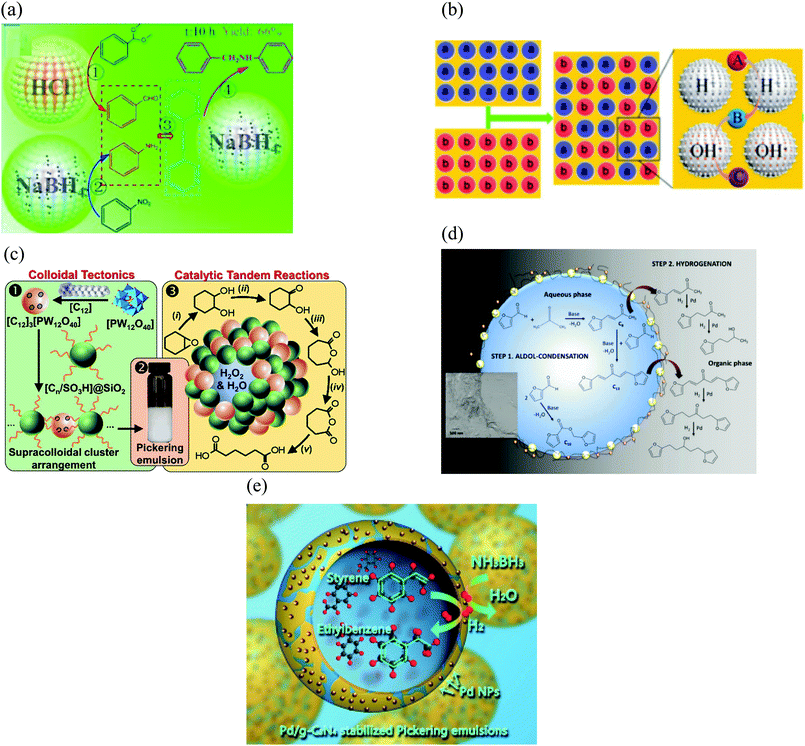 | ||
| Fig. 7 Examples of various one-pot cascade reactions. (a) Schematic illustration of the four-step cascade reaction for the synthesis of N-alkyl aniline. Partially hydrophobic silica nanospheres (modified with methyltrimethoxysilane) were used as the emulsifier while HCl and NaBH4 are the catalysts in the different steps. Reproduced from ref. 66 with permission from the American Chemical Society. (b) a and b represent acid-containing and base-containing reinforced w/o Pickering droplets, respectively. In this system the substrate A is transformed through an intermediate B to a final product C. Silica nanospheres modified with methyltrimethoxysilane are used as the solid emulsifier. Reproduced from ref. 67 with permission from The Royal Society of Chemistry. (c) Cascade reactions for the synthesis of adipic acid using [C18/SO3H]@SiO2 and [C12]3[PW12O40] nanoparticles both as catalyst and emulsifier. Reproduced from ref. 73 with permission from The Royal Society of Chemistry. (d) Schematic representation of the aldol condensation and hydrogenation reactions taking place at the oil–water interface in w/o emulsions stabilized with single- or multi-wall carbon nanotubes grown in MgO. Reproduced from ref. 116 with permission from Springer. (e) Schematic illustration of ammonia borane hydrolysis combined with styrene hydrogenation in an o/w Pickering emulsion catalyzed by Pd/graphitic carbon nitride which also acts as the emulsifier. Reproduced from ref. 78 with permission from Wiley. | ||
An example of acid–base catalysis in multi-step cascade reactions is given by Xue and co-workers.67 The two w/o Pickering emulsions, each containing either H2SO4 or ethylenediamine (EDA), were reinforced through cross-linking with tetraethoxysilane prior to mixing them together. The good shell permeability of the reinforced droplets ensures contact of the reactants/intermediate with the acid/base catalysts. Moreover, due to the high stability of the reinforced droplets, the lamination procedure used in ref. 66 could be avoided completely and the two emulsions were simply mixed together as shown in Fig. 7(b). A deacetalization-Knoevenagel condensation cascade reaction was investigated. When the two w/o Pickering emulsions without reinforcing were mixed together only 30% of benzaldehyde dimethylacetal was converted to the intermediate benzaldehyde and the final product was approximately 20%. However, when the emulsion droplets were reinforced, benzaldehyde dimethylacetal was fully transformed to the final product in the same period of time.
Yang et al. explored the synthesis of adipic acid from one-pot oxidative cleavage of cyclohexene oxide with aqueous hydrogen peroxide in a w/o Pickering emulsion stabilized by the interfacial self-assembly between dodecyltrimethylammonium phosphotungstate nanoparticles and silica particles functionalized with alkyl and sulfonic acid groups.73 As shown in Fig. 7(c), this reaction comprises hydrolysis (i and v) and oxidation (ii–iv) steps and the synergy between both particles acts as an efficient promoter for adipic acid production.
Zapata et al. prepared metal clusters (Pd, Pt, Ni) anchored on nanohybrids composed of basic metal oxide nanoparticles fused into carbon nanotubes to stabilize w/o emulsions and catalyze an aldol-condensation reaction.116 In the first step, aldol-condensation of furfural and acetone produces compounds in the range of C8–C13 (Fig. 7(d)). These long chain products have low solubility in water so they migrate to the oil phase where the hydrogenation reaction takes place catalyzed by the metallized nanohybrids.116
Another advantage of using Pickering emulsions to carry out multiple reactions is shown in ref. 78 for the hydrogenation of double bonds. This transformation can be realized by direct hydrogenation with gaseous hydrogen but special safety measures need to be in place. Alternatively, H2 can be transferred to the target molecule from a liquid hydrogen donor, such as ammonia borane (NH3BH3). However, this method still has some drawbacks linked to its high cost and the fact that the H2 produced partly escapes resulting in a waste of reagent. However, as shown by Han et al., if these reactions take place in an o/w Pickering emulsion stabilized with Pd nanoparticles loaded onto graphitic carbon nitride sheets (g-C3N4), the hydrogen generated in the water phase can be immediately used to conduct the hydrogenation in the oil phase (Fig. 7(e)).78
Other examples of multi-step cascade reactions can be found in the literature, such as the acid-catalyzed transesterification of vegetable oils with methanol53 or the phenolic hydrodeoxygenation of vanillin.20 In the last example, the effect of the reaction temperature was evaluated on the extent of hydrogenation, hydrogenolysis and decarbonylation reactions, which gave rise to a range of different products and phase migration processes depending on the reaction conditions. Moreover, the chemoselectivity changed significantly with increasing temperature.
8. Future research
This review describes the advances being made regarding the use of Pickering emulsions to carry out catalytic reactions. Despite being a recent research field that emerged from the combination of different branches within chemistry (physical, organic, inorganic and biochemistry), many publications have appeared since the first report in 2010. The liquids comprising the emulsion include water, polar solvents, oil and ionic liquids giving rise to a wide range of emulsion types. Particles of different types have been used either as the Pickering emulsion stabilizer or as the surface-active support for various catalysts. The use of Janus particles, however, has not been explored extensively with only a few examples available to date. It would be interesting, for instance, to prepare Janus particles in which each face contains a different catalyst to carry out two different reactions inside and outside emulsion droplets. Moreover, further examples could be studied where reactions occur selectively in one of the phases of the emulsion. Despite the dramatic increase in interfacial area covered by surface-active particles after emulsification (which has an impact on the catalytic performance), to the best of our knowledge there is no study that investigates the intrinsic reduction of mass transfer across the interface caused by the particle layer. Although this might not be significant, particle porosity can play an important role in enhancing the diffusion of reactants across the interface. Hence, does an interface containing more than one non-porous particle layer display a lower conversion to that stabilized by a particle monolayer? More recently, Pickering emulsions have been used to carry out catalytic reactions in flow, demonstrating a higher catalytic efficiency compared to the static Pickering emulsion case as reactants can be continuously fed from the inlet, collecting the products from the outlet without the need for separation. This brings other interesting advantages in line with the Principles of Green Chemistry. The use of Pickering emulsions to carry out multi-step cascade reactions could potentially find application industrially, specifically for the synthesis of drugs, where various steps are required. Emulsion droplets can be used as compartments to separate the different reactions and hence avoid the separation and purification of intermediates. In addition, emulsions of different type (for instance o/w and w/o), each of them compartmentalising a different reaction, could be mixed together to carry simultaneous reactions. By combining the idea of carrying out multiple cascade reactions in Pickering emulsions with flow methodology, this new procedure could revolutionize the way the chemical industry has been working up to now. Finally, potential exists to use particle-stabilised multiple emulsions (e.g. o/w/o, w/o/w) in which two different particulate catalysts are employed, one adsorbed at inner droplet interfaces and the other at outer globule interfaces. Such emulsions may be vehicles for either simultaneous or consecutive chemical reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors kindly acknowledge funding by the H2020FETOPEN-2016-2017 program of the European Commission to support AMBR (Grant agreement number: 737266-ONE FLOW).References
- C. A. Busacca, D. R. Fandrick, J. J. Song and C. H. Senanayake, The growing impact of catalysis in the pharmaceutical industry, Adv. Synth. Catal., 2011, 353, 1825–1864 CrossRef CAS.
- J. Hagen, Industrial Catalysis: A Practical Approach, Wiley-VCH, Weinheim, 2006, pp. 1–14 Search PubMed.
- J. J. Berzelius, Sur un force jusqu’ici peu remarquée qui est probablement active dans la formation des composes organiques, Section on Vegetable Chemistry, Jahres-Bericht, 1835, 14, 237 Search PubMed.
- W. Ostwald, Definition der Katalyse, Z. Phys. Chem., 1894, 15, 705–706 Search PubMed.
- P. T. Anastas and J. C. Warner, Green chemistry: theory and practice, Oxford University Press, Oxford, 1998 Search PubMed.
- S. Shylesh, V. Schünemann and W. R. Thiel, Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS.
- A. Z. Fadhel, P. Pollet, C. L. Liotta and C. A. Eckert, Combining the benefits of homogeneous and heterogeneous catalysis with tunable solvents and near critical water, Molecules, 2010, 15, 8400–8424 CrossRef CAS.
- S. L. Regen, Triphase catalysis, J. Am. Chem. Soc., 1975, 97, 5956–5957 CrossRef CAS.
- S. Ikeda, H. Nur, T. Sawadaishi, K. Ijiro, M. Shimomura and B. Ohtani, Direct observation of bimodal amphiphilic surface structures of zeolite particles for a novel liquid-liquid phase boundary catalysis, Langmuir, 2001, 17, 7976–7979 CrossRef CAS.
- K. Ikeue, S. Ikeda, A. Watanabe and B. Ohtani, Elucidation of the local structure of active titanium(IV) sites on silica-based phase-boundary catalysts for alkene epoxidation with aqueous hydrogen peroxide, Phys. Chem. Chem. Phys., 2004, 6, 2523–2528 RSC.
- K.-M. Choi, S. Ikeda, S. Ishino, K. Ikeue, M. Matsumura and B. Ohtani, Oxidation of hydrophobic alcohols using aqueous hydrogen peroxide over amphiphilic silica particles loaded with titanium(IV) oxide as a liquid-liquid phase-boundary catalyst, Appl. Catal., A, 2005, 278, 269–274 CrossRef CAS.
- T. Hashimoto and K. Maruoka, Recent development and application of chiral phase-transfer catalysts, Chem. Rev., 2007, 107, 5656–5682 CrossRef CAS.
- S. Shirakawa and K. Maruoka, Recent developments in asymmetric phase-transfer reactions, Angew. Chem., Int. Ed., 2013, 52, 4312–4348 CrossRef CAS.
- M. Palmer and H. Hatley, The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review, Water Res., 2018, 147, 60–72 CrossRef CAS.
- L. Wei, M. Zhang, X. Zhang, H. Xin and H. Yang, Pickering emulsion as an efficient platform for enzymatic reactions without stirring, ACS Sustainable Chem. Eng., 2016, 4, 6838–6843 CrossRef CAS.
- W. Zhang, L. Fu and H. Yang, Micrometer-scale mixing with Pickering emulsions: biphasic reactions without stirring, ChemSusChem, 2014, 7, 391–396 CrossRef CAS.
- B. P. Binks and T. S. Horozov, in Colloidal Particles at Liquid Interfaces, ed. B. P. Binks and T. S. Horozov, Cambridge University Press, Cambridge, 2006, ch. 1 Search PubMed.
- R. Aveyard, B. P. Binks and J. H. Clint, Emulsions stabilised solely by colloidal particles, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef CAS.
- M. Pera-Titus, L. Leclercq, J.-M. Clacens, F. De Campo and V. Nardello-Rataj, Pickering interfacial catalysis for biphasic systems: From emulsion design to green reactions, Angew. Chem., Int. Ed., 2015, 54, 2006–2021 CrossRef CAS.
- S. Crossley, J. Faria, M. Shen and D. E. Resasco, Solid nanoparticles that catalyze biofuel upgrade reactions at the water/oil interface, Science, 2010, 327, 68–72 CrossRef CAS.
- Y. Zhang, M. Zhang and H. Yang, Tuning biphasic catalysis reaction with a Pickering emulsion strategy exemplified by selective hydrogenation of benzene, ChemCatChem, 2018, 10, 5224–5230 CrossRef CAS.
- H. Jiang, Y. Li, L. Hong and T. Ngai, Submicron inverse Pickering emulsions for highly efficient and recyclable enzymatic catalysis, Chem. – Asian J., 2018, 13, 3533–3539 CrossRef CAS.
- Y. Li, G. Zhao, B. Hong, S. Zhao, X. Han and M. Pera-Titus, Unraveling particle size and roughness effects on the interfacial catalytic properties of Pickering emulsions, Colloids Surf., A, 2020, 599, 124800 CrossRef CAS.
- S. Yu, D. Zhang, J. Jiang, Z. Cui, W. Xia, B. P. Binks and H. Yang, Biphasic biocatalysis using a CO2-switchable Pickering emulsion, Green Chem., 2019, 21, 4062–4068 RSC.
- M. Wang, M. Wang, S. Zhang and J. Chen, Pickering gel emulsion stabilized by enzyme immobilized polymeric nanoparticles: a robust and recyclable biocatalyst system for biphasic catalysis, React. Chem. Eng., 2019, 4, 1459–1465 RSC.
- P. Finkle, H. D. Draper and J. H. Hildebrand, The theory of emulsification, J. Am. Chem. Soc., 1923, 45, 2780–2788 CrossRef CAS.
- J. H. Schulman and J. Leja, Control of contact angles at the oil-water-solid interfaces. Emulsions stabilized by solid particles (BaSO4), Trans. Faraday Soc., 1954, 50, 598–605 RSC.
- B. P. Binks and S. O. Lumsdon, Influence of particle wettability on the type and stability of surfactant-free emulsions, Langmuir, 2000, 16, 8622–8631 CrossRef CAS.
- A. M. Bago Rodriguez, L. Schober, A. Hinzmann, H. Gröger and B. P. Binks, Effect of particle wettability and particle concentration on the enzymatic dehydration of n-octanaloxime in Pickering emulsions, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202013171.
- Y. Zhang, R. Ettelaie, B. P. Binks and H. Yang, Highly selective catalysis at the liquid–liquid interface micro-region, ACS Catal., 2020 Search PubMed , submitted.
- M. Iqbal, Y. Tao, S. Xie, Y. Zhu, D. Chen, X. Wang, L. Huang, D. Peng, A. Sattar, M. A. B. Shabbir, H. I. Hussain, S. Ahmed and Z. Yuan, Aqueous two-phase system (ATPS): an overview and advances in its applications, Biol. Proced. Online, 2016, 18, 18 CrossRef.
- J. F. B. Pereira, M. G. Freire and J. A. P. Coutinho, Aqueous two-phase systems: Towards novel and more disruptive applications, Fluid Phase Equilib., 2020, 505, 112341 CrossRef CAS.
- C. C. de Oliveira, J. S. d. R Coimbra, A. D. G. Zuniga, J. P. Martins and A. M. d. O. Siqueira, Interfacial tension of aqueous two-phase systems containing poly(ethylene glycol) and potassium phosphate, J. Chem. Eng. Data, 2012, 57, 1648–1652 CrossRef.
- E. Scholten, L. M. C. Sagis and E. van der Linden, Bending rigidity of interfaces in aqueous phase-separated biopolymer mixtures, J. Phys. Chem. B, 2004, 108, 12164–12169 CrossRef CAS.
- Y. Chao and H. C. Shum, Emerging aqueous two-phase systems: from fundamentals of interfaces to biomedical applications, Chem. Soc. Rev., 2020, 49, 114–142 RSC.
- L.-H. Xue, C.-Y. Xie, S.-X. Meng, R.-X. Bai, X. Yang, Y. Wang, S. Wang, B. P. Binks, T. Guo and T. Meng, Polymer-protein conjugate particles with biocatalytic activity for stabilization of water-in-water emulsions, ACS Macro Lett., 2017, 6, 679–683 CrossRef CAS.
- F. P. Cakmak and C. D. Keating, Combining catalytic microparticles with droplets formed by phase coexistence: adsorption and activity of natural clays at the aqueous/aqueous interface, Sci. Rep., 2017, 7, 3215 CrossRef.
- D. C. Dewey, C. A. Strulson, D. N. Cacace, P. C. Bevilacqua and C. D. Keating, Bioreactor droplets from liposome-stabilized all-aqueous emulsions, Nat. Commun., 2014, 5, 4670 CrossRef CAS.
- D. N. Cacace, A. T. Rowland, J. J. Stapleton, D. C. Dewey and C. D. Keating, Aqueous emulsion droplets stabilized by lipid vesicles as microcompartments for biomimetic mineralization, Langmuir, 2015, 31, 11329–11338 CrossRef CAS.
- W. M. Aumiller, B. W. Davis, N. Hashemian, C. Maranas, A. Armaou and C. D. Keating, Coupled enzyme reactions performed in heterogeneous reaction media: experiments and modeling for glucose oxidase and horseradish peroxidase in PEG/citrate aqueous two-phase system, J. Phys. Chem. B, 2014, 118, 2506–2517 CrossRef CAS.
- C. A. Strulson, R. C. Molden, C. D. Keating and P. C. Bevilacqua, RNA catalysis through compartmentalization, Nat. Chem., 2012, 4, 941–946 CrossRef CAS.
- D. N. Cacace and C. D. Keating, Biocatalyzed mineralization in an aqueous two-phase system: effect of background polymers and enzyme partitioning, J. Mater. Chem. B, 2013, 1, 1794–1803 RSC.
- X. Tang, Y. Hou, Q. B. Meng, G. Zhang, F. Liang and X.-M. Song, Heteropoly acids-functionalized Janus particles as catalytic emulsifier for heterogeneous acylation in flow ionic liquid-in-oil Pickering emulsion, Colloids Surf., A, 2019, 570, 191–198 CrossRef CAS.
- W. Kunz and K. Häckl, The hype with ionic liquids as solvents, Chem. Phys. Lett., 2016, 661, 6–12 CrossRef CAS.
- Z. Meng, M. Zhang and H. Yang, Pickering emulsion droplets hosting ionic liquid catalysts for continuous-flow cyanosilylation reaction, Green Chem., 2019, 21, 627–633 RSC.
- X. Zhang, Y. Hou, R. Ettelaie, R. Guan, M. Zhang, Y. Zhang and H. Yang, Pickering emulsion-derived liquid-solid hybrid catalyst for bridging homogeneous and heterogeneous catalysis, J. Am. Chem. Soc., 2019, 141, 5220–5230 CrossRef CAS.
- M. Zhang, R. Ettelaie, T. Yan, S. Zhang, F. Cheng, B. P. Binks and H. Yang, Ionic liquid droplet microreactor for catalysis reactions not at equilibrium, J. Am. Chem. Soc., 2017, 139, 17387–17396 CrossRef CAS.
- L. Tao, M. Zhong, J. Chen, S. Jayakumar, L. Liu, H. Li and Q. Yang, Heterogeneous hydroformylation of long-chain alkenes in IL-in-oil Pickering emulsion, Green Chem., 2018, 20, 188–196 RSC.
- T. Meng, R. Bai, W. Wang, X. Yang, T. Guo and Y. Wang, Enzyme-loaded mesoporous silica particles with tuning wettability as a Pickering catalyst for enhancing biocatalysis, Catalysts, 2019, 9, 78 CrossRef.
- Z. Zhu, H. Tan, J. Wang, S. Yu and K. Zhou, Hydrodeoxygenation of vanillin as a bio-oil model over carbonaceous microspheres-supported Pd catalysts in the aqueous phase and Pickering emulsions, Green Chem., 2014, 16, 2636–2643 RSC.
- M. Xu, F. Richard, M. Corbet, P. Marion and J.-M. Clacens, Pickering emulsions assisted synthesis of fatty acetal over phenyl sulfonic groups grafted on activated charcoal, Appl. Catal., A, 2020, 597, 117543 CrossRef CAS.
- H. Shi, Z. Fan, V. Ponsinet, R. Sellier, H. Liu, M. Pera-Titus and J.-M. Clacens, Glycerol/dodecanol double Pickering emulsions stabilized by polystyrene-grafted silica nanoparticles for interfacial catalysis, ChemCatChem, 2015, 7, 3229–3233 CrossRef CAS.
- B. Yang, L. Leclercq, J.-M. Clacens and V. Nardello-Rataj, Acidic/amphiphilic silica nanoparticles: new eco-friendly Pickering interfacial catalysis for biodiesel production, Green Chem., 2017, 19, 4552–4562 RSC.
- A. Heyse, C. Plikat, M. Ansorge-Schumacher and A. Drews, Continuous two-phase biocatalysis using water-in-oil Pickering emulsions in a membrane reactor: Evaluation of different nanoparticles, Catal. Today, 2019, 331, 60–67 CrossRef CAS.
- M. Zhang, L. Wei, H. Chen, Z. Du, B. P. Binks and H. Yang, Compartmentalized droplets for continuous flow liquid-liquid interface catalysis, J. Am. Chem. Soc., 2016, 138, 10173–10183 CrossRef CAS.
- C. Wu, S. Bai, M. B. Ansorge-Schumacher and D. Wang, Nanoparticle cages for enzyme catalysis in organic media, Adv. Mater., 2011, 23, 5694–5699 CrossRef CAS.
- Y. Qu, R. Huang, W. Qi, Q. Qu, R. Su and Z. He, Structural insight into stabilization of Pickering emulsions with Fe3O4@SiO2 nanoparticles for enzyme catalysis in organic media, Part. Part. Syst. Charact., 2017, 34, 1700117 CrossRef.
- Z. Wang, M. C. M. van Oers, F. P. J. T. Rutjes and J. C. M. van Hest, Polymersome colloidosomes for enzyme catalysis in a biphasic system, Angew. Chem., Int. Ed., 2012, 51, 10746–10750 CrossRef CAS.
- Y. Jiang, X. Liu, Y. Chen, L. Zhou, Y. He, L. Ma and J. Gao, Pickering emulsion stabilized by lipase-containing periodic mesoporous organosilica particles: A robust biocatalyst system for biodiesel production, Bioresour. Technol., 2014, 153, 278–283 CrossRef CAS.
- Y. Zhao, X. Zhang, J. Sanjeevi and Q. Yang, Hydroformylation of 1-octene in Pickering emulsion constructed by amphiphilic mesoporous silica nanoparticles, J. Catal., 2016, 334, 52–59 CrossRef CAS.
- J. Liu, G. Lan, J. Peng, Y. Li, C. Li and Q. Yang, Enzyme confined in silica-based nanocages for biocatalysis in a Pickering emulsion, Chem. Commun., 2013, 49, 9558–9560 RSC.
- L. Wang, X. Liu, Y. Jiang, P. Liu, L. Zhou, L. Ma, Y. He, H. Li and J. Gao, Silica nanoflowers-stabilized Pickering emulsion as a robust biocatalysis platform for enzymatic production of biodiesel, Catalysts, 2019, 9, 1026 CrossRef CAS.
- P. A. Zapata, J. Faria, M. P. Ruiz, R. E. Jentoft and D. E. Resasco, Hydrophobic zeolites for biofuel upgrading reactions at the liquid-liquid interface in water/oil emulsions, J. Am. Chem. Soc., 2012, 134, 8570–8578 CrossRef CAS.
- X. Du and S. Z. Qiao, Dendritic silica particles with center-radial pore channels: Promising platforms for catalysis and biomedical applications, Small, 2015, 11, 392–413 CrossRef CAS.
- H. Chen, H. Zou, Y. Hao and H. Yang, Flow Pickering emulsion interfaces enhance catalysis efficiency and selectivity for cyclization of citronellal, ChemSusChem, 2017, 10, 1989–1995 CrossRef CAS.
- H. Yang, L. Fu, L. Wei, J. Liang and B. P. Binks, Compartmentalization of incompatible reagents within Pickering emulsion droplets for one-pot cascade reactions, J. Am. Chem. Soc., 2015, 137, 1362–1371 CrossRef CAS.
- N. Xue, G. Zhang, X. Zhang and H. Yang, A reinforced Pickering emulsion for cascade reactions, Chem. Commun., 2018, 54, 13014–13017 RSC.
- D. Stehl, N. Milojević, S. Stock, R. Schomäcker and R. von Klitzing, Synergistic effects of a Rhodium catalyst on particle-stabilized Pickering emulsions for the hydroformylation of a long-chain olefin, Ind. Eng. Chem. Res., 2019, 58, 2524–2536 CrossRef CAS.
- J. Huang and H. Yang, A pH-switched Pickering emulsion catalytic system: high reaction efficiency and facile catalyst recycling, Chem. Commun., 2015, 51, 7333–7336 RSC.
- W.-J. Zhou, L. Fang, Z. Fan, B. Albela, L. Bonneviot, F. De Campo, M. Pera-Titus and J.-M. Clacens, Tunable catalysts for solvent-free biphasic systems: Pickering interfacial catalysts over amphiphilic silica nanoparticles, J. Am. Chem. Soc., 2014, 136, 4869–4872 CrossRef CAS.
- P. Hao, D. K. Schwartz and J. W. Medlin, Effect of surface hydrophobicity of Pd/Al2O3 on vanillin hydrodeoxygenation in a water/oil system, ACS Catal., 2018, 8, 11165–11173 CrossRef CAS.
- B. Xue, T. Xu, D. Li, J. Xu, Y. Li, F. Wang and J. Zhu, A Pickering emulsion of a bifunctional interface prepared from Pd nanoparticles supported on silicane-modified graphene oxide: an efficient catalyst for water-mediated catalytic hydrogenation, Catal. Sci. Technol., 2020, 10, 1096–1105 RSC.
- B. Yang, L. Leclercq, V. Schmitt, M. Pera-Titus and V. Nardello-Rataj, Colloidal tectonics for tandem synergistic Pickering interfacial catalysis: oxidative cleavage of cyclohexene oxide into adipic acid, Chem. Sci., 2019, 10, 501–507 RSC.
- X. Yang, Y. Liang, Y. Cheng, W. Song, X. Wang, Z. Wang and J. Qiu, Hydrodeoxygenation of vanillin over carbon nanotube-supported Ru catalysts assembled at the interfaces of emulsion droplets, Catal. Commun., 2014, 47, 28–31 CrossRef CAS.
- H. Yang, T. Zhou and W. Zhang, A strategy for separating and recycling solid catalysts based on the pH-triggered Pickering-emulsion inversion, Angew. Chem., Int. Ed, 2013, 52, 7455–7459 CrossRef CAS.
- N. Fessi, M. F. Nsib, Y. Chevalier, C. Guillard, F. Dappozze, A. Houas, L. Palmisano and F. Parrino, Photocatalytic degradation enhancement in Pickering emulsions stabilized by solid particles of bare TiO2, Langmuir, 2019, 35, 2129–2136 CrossRef CAS.
- Z. Han, L. Peng, Q. Li and Y. Hao, An interfacially active Pd/C catalyst enhanced hydrogenation of aromatic compounds in Pickering emulsion, Catal. Lett., 2017, 147, 1615–1621 CrossRef.
- C. Han, P. Meng, E. R. Waclawik, C. Zhang, X.-H. Li, H. Yang, M. Antonietti and J. Xu, Palladium/Graphitic carbon nitride (g-C3N4) stabilized emulsion microreactor as a store for hydrogen from ammonia borane for use in alkene hydrogenation, Angew. Chem., Int. Ed, 2018, 57, 14857–14861 CrossRef CAS.
- Y. Hao, Y. Liu, R. Yang, X. Zhang, J. Liu and H. Yang, A pH-responsive TiO2-based Pickering emulsion system for in situ catalyst recycling, Chin. Chem. Lett., 2018, 29, 778–782 CrossRef CAS.
- A. L. Sadgar, T. S. Deore and R. V. Jayaram, Pickering interfacial catalysis—Knoevenagel condensation in magnesium oxide-stabilized Pickering emulsion, ACS Omega, 2020, 5, 12224–12235 CrossRef CAS.
- F. Peng, J. Xu, X. Zeng, G. Feng and H. Bao, Metal-decorated Pickering emulsion for continuous flow catalysis, Part. Part. Syst. Charact., 2020, 37, 1900382 CrossRef CAS.
- J. H. Clark, Solid acids for green chemistry, Acc. Chem. Res., 2002, 35, 791–797 CrossRef CAS.
- N. Mohaghegh, M. Tasviri, E. Rahimi and M. R. Gholami, A novel p-n junction Ag3PO4/BiPO4 based stabilized Pickering emulsion for highly efficient photocatalysis, RSC Adv., 2015, 5, 12944–12955 RSC.
- C. Herrera, D. Fuentealba, I. T. Ghampson, C. Sepulveda, J. L. García-Fierro, R. I. Canales and N. Escalona, Selective conversion of biomass-derived furfural to cyclopentanone over carbon nanotube-supported Ni catalyst in Pickering emulsions, Catal. Commun., 2020, 144, 106092 CrossRef CAS.
- H. Su, C.-A. H. Price, L. Jing, Q. Tian, J. Liu and K. Qian, Janus particles: design, preparation, and biomedical applications, Mater. Today Bio., 2019, 4, 100033 CrossRef CAS.
- J. Faria, M. P. Ruiz and D. E. Resasco, Phase-selective catalysis in emulsions stabilized by Janus silica-nanoparticles, Adv. Synth. Catal., 2010, 352, 2359–2364 CrossRef CAS.
- C. Wang, E. Bu, Y. Chen, Z. Cheng, J. Zhang, R. Shu and Q. Song, Enhanced photoreforming hydrogen production: Pickering interfacial catalysis from a bio-derived biphasic system, Renewable Energy, 2019, 134, 113–124 CrossRef CAS.
- D. Li, J. Jiang and C. Cai, Palladium nanoparticles anchored on amphiphilic Janus-type cellulose nanocrystals for Pickering interfacial catalysis, Chem. Commun., 2020, 56, 9396–9399 RSC.
- A. M. Bago Rodriguez and B. P. Binks, Capsules from Pickering emulsion templates, Curr. Opin. Colloid Interface Sci., 2019, 44, 107–129 CrossRef CAS.
- X. Yang, Y. Wang, R. Bai, H. Ma, W. Wang, H. Sun, Y. Dong, F. Qu, Q. Tang, T. Guo, B. P. Binks and T. Meng, Pickering emulsion-enhanced interfacial biocatalysis: tailored alginate microparticles act as particulate emulsifier and enzyme carrier, Green Chem., 2019, 21, 2229–2233 RSC.
- T. Nallamilli, B. P. Binks, E. Mani and M. G. Basavaraj, Stabilization of Pickering emulsions with oppositely charged latex particles: Influence of various parameters and particle arrangement around droplets, Langmuir, 2015, 31, 11200–11208 CrossRef CAS.
- B. P. Binks, W. Liu and J. A. Rodrigues, Novel stabilisation of emulsions via the heteroaggregation of nanoparticles, Langmuir, 2008, 24, 4443–4446 CrossRef CAS.
- T. Nallamilli, E. Mani and M. G. Basavaraj, A model for the prediction of droplet size in Pickering emulsions stabilized by oppositely charged particles, Langmuir, 2014, 30, 9336–9345 CrossRef CAS.
- W. Richtering, Responsive emulsions stabilized by stimuli-sensitive microgels: emulsions with special non-Pickering properties, Langmuir, 2012, 28, 17218–17229 CrossRef CAS.
- M. Destribats, M. Rouvet, C. Gehin-Delval, C. Schmitt and B. P. Binks, Emulsions stabilised by whey protein microgel particles: towards food-grade Pickering emulsions, Soft Matter, 2014, 10, 6941–6954 RSC.
- A. M. Bago Rodriguez, B. P. Binks and T. Sekine, Emulsion stabilisation by complexes of oppositely charged synthetic polyelectrolytes, Soft Matter, 2018, 14, 239–254 RSC.
- A. M. Bago Rodriguez, B. P. Binks and T. Sekine, Emulsions stabilized with polyelectrolyte complexes prepared from a mixture of a weak and a strong polyelectrolyte, Langmuir, 2019, 35, 6693–6707 CAS.
- L. Peng, A. Feng, S. Liu, M. Huo, T. Fang, K. Wang, Y. Wei, X. Wang and J. Yuan, Electrochemical stimulated Pickering emulsion for recycling of enzyme in biocatalysis, ACS Appl. Mater. Interfaces, 2016, 8, 29203–29207 CrossRef CAS.
- S. Wiese, A. C. Spiess and W. Richtering, Microgel-stabilized smart emulsions for biocatalysis, Angew. Chem., Int. Ed, 2013, 52, 576–579 CrossRef CAS.
- H. Jiang, L. Liu, Y. Li, S. Yin and T. Ngai, Inverse Pickering emulsion stabilized by binary particles with contrasting characteristics and functionality for interfacial biocatalysis, ACS Appl. Mater. Interfaces, 2020, 12, 4989–4997 CrossRef CAS.
- X.-M. Huang, Z.-J. Luo, J. Guo, Q.-J. Ruan, J.-M. Wang and X. Yang, Enzyme-adsorbed chitosan nanogel particles as edible Pickering interfacial biocatalysts and lipase-responsive phase inversion of emulsions, J. Agric. Food Chem., 2020, 68, 8890–8899 CrossRef CAS.
- Microgel Suspensions: Fundamentals and Applications, ed. A. Fernandez-Nieves, H. Wyss, J. Mattsson and D. A. Weitz, Wiley-VCH, New York, 2011, pp. 3–4 Search PubMed.
- R. W. Style, L. Isa and E. R. Dufresne, Adsorption of soft particles at fluid interfaces, Soft Matter, 2015, 11, 7412–7419 RSC.
- C. Deng, M. Xu, Z. Dong, L. Li, J. Yang, X. Guo, L. Peng, N. Xue, Y. Zhu and W. Ding, Exclusively catalytic oxidation of toluene to benzaldehyde in an O/W emulsion stabilized by hexadecylphosphate acid terminated mixed-oxide nanoparticles, Chin. J. Catal., 2020, 41, 341–349 CrossRef CAS.
- X. Yang, D. Li, J. Zhai, F. Wang, B. Xue, J. Zhu and Y. Li, Pickering emulsion prepared by bi-functional graphene oxide as efficient catalyst for aqueous nucleophilic substitution reactions, Colloids Surf., A, 2020, 585, 124138 CrossRef CAS.
- K. Chen and F. H. Arnold, Engineering new catalytic activities in enzymes, Nat. Catal., 2020, 3, 203–213 CrossRef CAS.
- Z. Sun, U. Glebe, H. Charan, A. Böker and C. Wu, Enzyme-polymer conjugates as robust Pickering interfacial biocatalysts for efficient biotransformations and one-pot cascade reactions, Angew. Chem., Int. Ed., 2018, 57, 13810–13814 CrossRef CAS.
- J. Shi, X. Wang, S. Zhang, L. Tang and Z. Jiang, Enzyme-conjugated ZIF-8 particles as efficient and stable Pickering interfacial biocatalysts for biphasic biocatalysis, J. Mater. Chem. B, 2016, 4, 2654–2661 RSC.
- Z. Chen, H. Ji, C. Zhao, E. Ju, J. Ren and X. Qu, Individual surface-engineered microorganisms as robust Pickering interfacial biocatalysts for resistance-minimized phase-transfer bioconversion, Angew. Chem., Int. Ed., 2015, 54, 4904–4908 CrossRef CAS.
- Z. Chen, C. Zhao, E. Ju, H. Ji, J. Ren, B. P. Binks and X. Qu, Design of surface-active artificial enzyme particles to stabilize Pickering emulsions for high-performance biphasic biocatalysis, Adv. Mater., 2016, 28, 1682–1688 CrossRef CAS.
- X. Zhang and P. Zhang, Polymersomes in nanomedicine – A review, Curr. Med. Chem., 2017, 13, 124–129 CAS.
- H. Tan, P. Zhang, L. Wang, D. Yang and K. Zhou, Multifunctional amphiphilic carbonaceous microcapsules catalyze water/oil biphasic reactions, Chem. Commun., 2011, 47, 11903–11905 RSC.
- Z. Fang, D. Yang, Y. Gao and H. Li, pH-responsible Pickering emulsion and its catalytic application for reaction at water–oil interface, Colloid Polym. Sci., 2015, 293, 1505–1513 CrossRef CAS.
- J. Tang, S. Cao and J. Wang, CO2-switchable Pickering emulsions: efficient and tunable interfacial catalysis for alcohol oxidation in biphasic systems, Chem. Commun., 2019, 55, 11079–11082 RSC.
- Y. Xi, B. Liu, H. Jiang, S. Yin, T. Ngai and X. Yang, Sodium caseinate as a particulate emulsifier for making indefinitely recycled pH-responsive emulsions, Chem. Sci., 2020, 11, 3797–3803 RSC.
- P. A. Zapata, J. Faria, M. P. Ruiz and D. E. Resasco, Condensation/hydrogenation of biomass-derived oxygenates in water/oil emulsions stabilized by nanohybrid catalysts, Top. Catal., 2012, 55, 38–52 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sm01636e |
| This journal is © The Royal Society of Chemistry 2020 |