Polymeric hybrid aerogels and their biomedical applications
Abstract
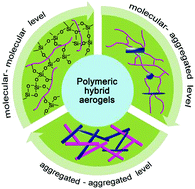
Aerogels are a class of porous materials that possess extremely high specific surface area, high pore volume, high porosity, and variable chemical structures. They have been widely applied in the fields of aerospace, chemical engineering, construction, electrotechnics, and biomedicine. In recent years a great boom in aerogels has been observed, where various new aerogels with novel physicochemical properties and functions have been synthesized. Nevertheless, native aerogels with a single component normally face severe problems such as low mechanical strength and lack of functions. One strategy to solve the problems is to construct hybrid aerogels. In this study, a comprehensive review on polymer based hybrid aerogels is presented, including polymer–polymer, polymer–carbon material, and polymer–inorganic hybrid aerogels, which will be introduced and discussed in view of their chemical structures and hybrid structures. Most importantly, polymeric hybrid aerogels are classified into three different composition levels, which are molecular-level, molecular-aggregate-level, and aggregate-level, due to the fact that hybrid aerogels with the same chemical structures but with different composition levels might show quite different functions or properties. The biomedical applications of these hybrid aerogels will also be reviewed and discussed, where the polymeric components in the hybrid aerogels provide the main contribution. This review would provide creative design principles for aerogels by considering both their chemical and physical structures.


 Please wait while we load your content...
Please wait while we load your content...