Bi-functional peptide-based 3D hydrogel-scaffolds†
Abstract
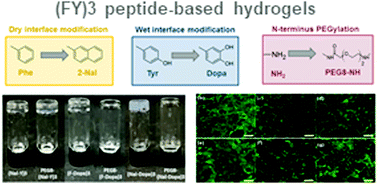
Over the last few years, hydrogels have been proposed for many biomedical applications, including drug delivery systems and scaffolds for tissue engineering. In particular, peptides have been envisioned as excellent candidates for the development of hydrogel materials, due to their intrinsic biocompatibility, ease of handling, and intrinsic biodegradability. Recently, we developed a novel hybrid polymer–peptide conjugate, PEG8-(FY)3, which is able to self-assemble into a self-supporting soft hydrogel over dry and wet surfaces as demonstrated by molecular dynamics simulation. Here, we describe the synthesis and supramolecular organization of six novel hexapeptides rationally designed by punctual chemical modification of the primary peptide sequence of the ancestor peptide (FY)3. Non-coded amino acids were incorporated by replacing the phenylalanine residue with naphthylalanine (Nal) and tyrosine with dopamine (Dopa). We also studied the effect of the modification of the side chain and the corresponding PEGylated peptide analogues, on the structural and mechanical properties of the hydrogel. Secondary structure, morphology and rheological properties of all the peptide-based materials were assessed by various biophysical tools. The in vitro biocompatibility of the supramolecular nanostructures was also evaluated on fibroblast cell lines. We conclude that the PEG8-(Nal-Dopa)3 hydrogel possesses the right properties to serve as a scaffold and support cell growth.
- This article is part of the themed collection: Peptide Soft Materials


 Please wait while we load your content...
Please wait while we load your content...