Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Filler size effect in an attractive fibrillated network: a structural and rheological perspective†
Vincenzo
Calabrese‡
 *a,
Marcelo A.
da Silva
*a,
Marcelo A.
da Silva
 a,
Lionel
Porcar
b,
Saffron J.
Bryant
a,
Lionel
Porcar
b,
Saffron J.
Bryant
 a,
Kazi M. Zakir
Hossain
a,
Janet L.
Scott
a,
Kazi M. Zakir
Hossain
a,
Janet L.
Scott
 ac and
Karen J.
Edler
ac and
Karen J.
Edler
 *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: vc355@bath.ac.uk; 355calabrese@gmail.com; k.edler@bath.ac.uk
bInstitut Laue-Langevin, 71 avenue des Martyrs, CS 20156, 38042 Grenoble cedex 9, France
cCentre for Sustainable Chemical Technologies, University of Bath, Claverton Down, Bath, BA2 7AY, UK
First published on 2nd March 2020
Abstract
The effect of the filler size on the structural and mechanical properties of an attractive fibrillated network composed of oxidised cellulose nanofibrils (OCNF) in water was investigated. Silica nanoparticles with a diameter of ca. 5 nm (SiNp5) and and ca. 158 nm (SiNp158) were chosen as non-interacting fillers of the OCNF network. These filler sizes were chosen, respectively, to have a particle size which was either similar to that of the network mesh size or much larger than it. Contrast matched small angle neutron scattering (SANS) experiments revealed that the presence of the fillers (SiNp5 and SiNp158) did not perturb the structural properties of the OCNF network at the nanometer scale. However, the filler size difference strongly affected the mechanical properties of the hydrogel upon large amplitude oscillatory shear. The presence of the smaller filler, SiNp5, preserved the mechanical properties of the hydrogels, while the larger filler, SiNp158, allowed a smoother breakage of the network and low network recoverability after breakage. This study showed that the filler-to-mesh size ratio, for non-interacting fillers, is pivotal for tailoring the non-linear mechanical properties of the gel, such as yielding and flow.
1 Introduction
The growing need for sustainable materials has promoted interest in the use of cellulose nanofibrils (CNF) as building blocks for renewable products. CNF belong to the colloidal domain and are usually characterized by a radius in the order of a few nanometers and a length of 100–1000 nm depending on the preparation method and source.1,2 TEMPO-mediated oxidation of cellulose has been, for instance, a successful route for the production of oxidised cellulose nanofibrils (OCNF) on a large scale.2–4 Importantly, the carboxylate groups on the OCNF surface yield a surface negative charge which allows the preparation of stable aqueous dispersions.3 Interfibrillar repulsive/attractive forces have been reported to be strongly dependent on the pH5,6 and ionic strength7–9 of the aqueous media.10 Charge screening due to counterion binding to the carboxylate group has been shown to be an effective gelation mechanism.7,9 Structural investigation of OCNF dispersions, via small angle X-ray scattering, revealed that, for concentrations above the overlap threshold,11 a clear transition from a repulsive to an attractive fibrillated network occurs upon increasing the ionic strength.9 This finding is directly related to the sol–gel transition9 and to the theoretical expectations based on the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory for two cylindrical rods.7 To broaden the understanding of interfibrillar assembly in systems closer to industrially relevant scenarios, the use of additives, such as surfactants11,12 and polymers,13,14 in cellulose nanoparticle-based hydrogels has been explored.1 Nevertheless, knowledge of the interplay between non-interacting colloidal fillers and a fibrillated network is lacking. The filler surface chemistry, size and volume fraction have been mainly investigated in protein-based hydrogels and are referred to as “active” or “inactive” fillers based on their ability to strengthen or weaken the gel, respectively.15–17 In principle, fillers could be employed to modulate other mechanical properties such as structural breakage (yielding), flow, structural recoverability and plasticity, which are important parameters to account for in processing operations and/or customer appreciation.18 Such mechanical properties often occur upon large deformations and high shear rates, where the mechanical response of the material depends on the applied strain and shear rate, identified as non-linear viscoelastic region (NLVR). On this ground, we investigated the filler size effect on the linear and non-linear viscoelastic response of an attractive fibrillated network composed of OCNF. The interfibrillar attractive forces were promoted via addition of 100 mM NaCl, leading to an OCNF network with a mesh size, defined as the average distance between the nearest fibrillar junction zones, of 20–40 nm as recently reported by our group.9 Silica nanoparticles (SiNp) with average dimensions similar to the mesh size of the network and SiNp larger (ca. by a factor of 5) than the network mesh size were chosen as non-interacting fillers in the OCNF-based gel. We show that the filler-to-mesh-size ratio, for non-interacting fillers, is of pivotal importance for tailoring non-linear mechanical properties of the gel such as yielding and flow.2 Experimental
2.1 Materials
An OCNF dispersion was prepared as previously described,19 using a TEMPO/NaOCl/NaBr oxidation method.20 A 2 wt% OCNF stock was redispersed from freeze-dried OCNF in ultrapure deionised (DI) water (18.2 MΩ cm) and stirred overnight, followed by a mild sonication process (Ultrasonic Processor FB-505, Fisher, 200 W cm−2, equipped with a 1 cm probe, using a series of 1 s on/1 s off in pulse mode for a net time of 120 s at 30% amplitude on ca. 45 mL dispersion contained in an ice bath). A 10 wt% SiNp158 dispersion was prepared by dispersing silica nanopowder (718483 Sigma-Aldrich®) in ultrapure DI water followed by sonication (as for the OCNF indicated above) and used without further treatment. A ca. 9 wt% aqueous SiNp5 dispersion, provided by Geo40™ (sodium stabilised colloidal silica of geothermal origin), was dialysed using cellulose dialysis tubing (Sigma-Aldrich® cellulose dialysis tubing, molecular weight cut-off of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da) against DI water for 3 days, refreshing the DI water twice per day. The dialysed SiNp5 dispersion was pH adjusted to 7 using HCl (aq) and further dialysed against DI water, as previously described. Larger aggregates were removed using a syringe filter unit with a cut-off size of 0.22 μm (Millex®-GS), and the solid content (wt%) of the filtered dispersion was obtained gravimetrically. Specific OCNF, OCNF–SiNp5 and OCNF–SiNp158 concentrations were obtained via dilution of the stock dispersions described above, except where otherwise stated, followed by the addition of a 2 M NaCl solution to achieve a final NaCl concentration of 100 mM in all samples.
400 Da) against DI water for 3 days, refreshing the DI water twice per day. The dialysed SiNp5 dispersion was pH adjusted to 7 using HCl (aq) and further dialysed against DI water, as previously described. Larger aggregates were removed using a syringe filter unit with a cut-off size of 0.22 μm (Millex®-GS), and the solid content (wt%) of the filtered dispersion was obtained gravimetrically. Specific OCNF, OCNF–SiNp5 and OCNF–SiNp158 concentrations were obtained via dilution of the stock dispersions described above, except where otherwise stated, followed by the addition of a 2 M NaCl solution to achieve a final NaCl concentration of 100 mM in all samples.
2.2 Methods
Small angle X-ray scattering (SAXS) measurements were performed on an Anton-Paar SAXSpoint 2.0 equipped with a copper source (Cu Kα, λ = 1.542 Å) and a 2D EIGER R series Hybrid Photon Counting (HPC) detector. The sample-to-detector distance was 556 mm covering a range of the scattering vector (q) of about 0.01 < q < 0.4 Å−1. Samples were loaded into 1 mm quartz capillaries, and the scattering intensity (I(q)) was collected in three frames, with 300 s exposure per frame. Temperature was kept at 25 °C via a Peltier unit (±0.1 °C). Small angle neutron scattering (SANS) experiments were conducted at the Institut Laue Langevin (Grenoble, France) on the D22 SANS beamline using a wavelength of 6 Å and a sample-to-detector distance of 2.8 and 17.6 m to yield a q range of 0.003 < q < 0.4 Å−1. Temperature was kept at 25 °C via a Julabo circulating waterbath (±0.5 °C). Particles that have the same scattering length density (SLD) as the continuous phase do not contribute to the scattering intensity (I(q) = 0), and they are said to be contrast matched.21 We determined the contrast match point of the SiNp, using different H2O/D2O ratios as the continuous phase. This is possible due to the different SLD between H2O (−0.5 × 10−6 Å−2) and D2O (6.3 × 10−6 Å−2), which allows tuning of the SLD of the continuous phase upon changing the H2O/D2O ratios. Samples were prepared with a continuous phase composed of 60 vol% D2O (99.9 atom% D, Sigma-Aldrich®) and 40 vol% H2O (DI water), which was experimentally determined to be the contrast match point for SiNp (Fig. S1, ESI†) in agreement with what was previously reported.22 For this experiment, OCNF, OCNF–SiNp5 and OCNF–SiNp158 concentrations were obtained via dilution of 2 wt% freeze-dried OCNF dispersed in pure D2O (prepared as previously described) and SiNp stock dispersions described above (SiNp5 and SiNp158 (in H2O)) with the required H2O/D2O ratio, followed by the addition of a 2 M NaCl solution (in H2O) to achieve a final NaCl concentration of 100 mM in all samples. Samples were measured in 2 cm wide optical quartz cells with 1 mm path length. For SAXS and SANS measurements, background subtraction and data treatment were performed using the Irena package,23 whilst data analysis was done using the NIST SANS Analysis package (models were used without further modification),24 both within IGOR Pro (Wavemetrics, Inc.). Data are presented as I(q) vs. q and I(q) vs. d, where d is the d-spacing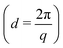 . Details regarding the model of non-interacting flexible cylinders with an elliptical cross-section are described by Schmitt et al.9
. Details regarding the model of non-interacting flexible cylinders with an elliptical cross-section are described by Schmitt et al.9
Dynamic light scattering (DLS) was performed on diluted samples (0.01 wt% in 100 mM NaCl) using a Malvern Zetasizer Nano ZSP® (Malvern, UK). The samples were loaded in disposable polystyrene cells with a path length of 1 cm and measured as an average of 4 measurements of 100 scans each. The values are reported as normalised intensity and obtained from the average of three separate samples. Temperature was kept at 25 °C (±0.1 °C).
The rheological measurements of the OCNF-based hydrogels were performed using a stress-controlled rheometer (Discovery HR3, TA instruments®) equipped with a sandblasted plate-plate geometry (40 mm). To avoid evaporation, the edge of the samples was covered with low viscosity mineral oil and further covered with a solvent trap to ensure constant temperature within the chamber (25 °C ± 0.1 °C via a Peltier unit). After loading, the gel was exposed to (i) a 30 s pre-shear at 300 s−1 to ensure equal sample history, (ii) a time sweep employing small strain amplitudes (γ0 = 0.05 (%)) at a constant angular frequency (ω = 1 rad s−1) for 50 × 103 s, (iii) a frequency sweep at a constant strain amplitude, γ0 = 0.1%, being in the linear viscoelastic region (LVR) (Fig. S2, ESI†) and (iv) a strain sweep at constant frequency, ω = 1 rad s−1. The storage and loss moduli, G′ and G′′, respectively, were computed by the TRIOS software and used to obtain tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′. For (iv), raw data were acquired as stress (σ) as a function of intracycle shear rate
δ = G′′/G′. For (iv), raw data were acquired as stress (σ) as a function of intracycle shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) (s−1) and the instantaneous intracycle strain (γ) and presented in the form of Lissajous plots as σ vs.
(s−1) and the instantaneous intracycle strain (γ) and presented in the form of Lissajous plots as σ vs. ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) and σ vs. γ, known as the viscous and the elastic projection, respectively.
and σ vs. γ, known as the viscous and the elastic projection, respectively.
3 Results and discussion
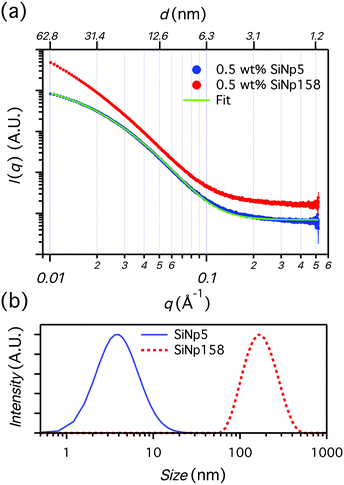
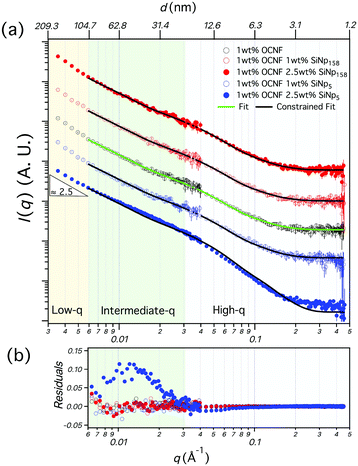
Gelation of the 1 wt% OCNF suspension was achieved upon addition of NaCl (100 mM), resulting in an attractive fibrillated network with a defined mesh size of 20–40 nm.9 Silica nanoparticles, with dimensions similar to and greater than the mesh size of the network, were incorporated in the 1 wt% OCNF gel, and their effect on structural and mechanical properties of the OCNF-based gel was investigated. Although silica nanoparticles were chosen to avoid strong interactions with OCNF (e.g. electrostatic, hydrophobic), experimental evidence for this was sought. At first, the filler sizes were evaluated via SAXS using conditions of ionic strength the same as the one employed in this study to induce gelation of OCNF (Fig. 1a). Contrary to SiNp158, SiNp5 displayed an approaching plateau at low q, indicating that the characteristic size of the object is probed (radius of gyration).25 The data of SiNp5 were fitted to a model of spheres with a log-normal size distribution, yielding a particle mean size of 5.2 ± 0.2 nm and a size distribution as shown in Fig. 1b. Since SAXS measurements did not access the larger size of SiNp158, DLS measurements were used to determine the hydrodynamic size of the particles (Fig. 1b). This accounts for the hydration shell of the particles which is expected to be of the order of a few nanometers at this ionic strength.26 Overall, SiNp158 displayed a higher mean size (158.0 ± 1.3 nm) and a narrower size distribution compared to SiNp5. To explore the effect of the filler on the structural properties of the OCNF network, SANS measurements were performed under conditions where the SiNp scattering contribution was matched to that of the solvent. This results in a dominant OCNF scattering intensity, allowing us to probe, solely, the structural properties of the OCNF network in the presence of SiNp. In practice, this was achieved using a continuous phase composed of a mixture of 60 vol% D2O and 40 vol% H2O (see Fig. S1 for contrast match point determination for SiNp, ESI†). According to previous reports, the scattering pattern of 1 wt% OCNF at 100 mM NaCl (Fig. 2a) contains information regarding the shape of the fibrils in the high-q and intermediate-q region, which probe the radius and the larger dimensions of the fibril, respectively.9,19,27 The upturn in the low-q region has been instead associated with the attractive interfibrillar interactions as described by Schmitt et al.9 The data were fitted, in the q region where interfibrillar interactions are not detectable (high-q and intermediate-q range), to a model of non-interacting flexible cylinders with an elliptical cross-section from which the minor radius (Rmin), major radius (Rmaj) and the Kuhn length (bKuhn) were obtained, where bKuhn is indicative of the OCNF mesh size.9 The data fitting was carried out using a fixed and arbitrary contour length (L) of 500 nm as the average length was not accessible in the probed q range, as indicated by the lack of a plateau in the low-q region. In addition, the interfibrillar interactions, in the low-q region, would not allow extrapolation of the OCNF contour length. Since the lower threshold for the data fitting was qmin = 0.06−1, using values of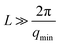 kept the fitting unchanged. The model yielded values of Rmin = 1.1 ± 0.1 nm, Rmaj = 4.7 ± 0.1 nm and bKuhn = 21.4 ± 0.1 nm, in good agreement with previous SAXS measurements9,19,27 and imaging analysis of OCNF.9 Upon SiNp addition, the SANS patterns had a similar trend to that of the pure OCNF gel, indicating that, in the probed q range, neither of these fillers strongly alters the structure of the fibrillated network. As such, the SiNp-containing samples were fitted using fixed values of Rmin = 1.1, Rmaj = 4.7 and bKuhn = 21.4 nm as found for OCNF, allowing only scale and background to vary. The data were satisfactorily fitted using these constrained values with the exception of the sample containing 2.5 wt% SiNp5, which displayed some difference in the intermediate-q region as shown by the residual plot (Fig. 2b). The poorer fitting in the intermediate-q range could be due to a small residual unmatched scattering contribution from SiNp5. Analysis of the low-q region revealed a slope of ≈2.5 for all the samples, indicating that neither of the fillers alters the attractive interactions between OCNF at this length scale. Overall, both fillers preserved the architecture of the fibrillated OCNF network at the nanometer length scale, enabling comparison of the rheological properties of the OCNF-based gels under conditions where the network architecture is the same.
kept the fitting unchanged. The model yielded values of Rmin = 1.1 ± 0.1 nm, Rmaj = 4.7 ± 0.1 nm and bKuhn = 21.4 ± 0.1 nm, in good agreement with previous SAXS measurements9,19,27 and imaging analysis of OCNF.9 Upon SiNp addition, the SANS patterns had a similar trend to that of the pure OCNF gel, indicating that, in the probed q range, neither of these fillers strongly alters the structure of the fibrillated network. As such, the SiNp-containing samples were fitted using fixed values of Rmin = 1.1, Rmaj = 4.7 and bKuhn = 21.4 nm as found for OCNF, allowing only scale and background to vary. The data were satisfactorily fitted using these constrained values with the exception of the sample containing 2.5 wt% SiNp5, which displayed some difference in the intermediate-q region as shown by the residual plot (Fig. 2b). The poorer fitting in the intermediate-q range could be due to a small residual unmatched scattering contribution from SiNp5. Analysis of the low-q region revealed a slope of ≈2.5 for all the samples, indicating that neither of the fillers alters the attractive interactions between OCNF at this length scale. Overall, both fillers preserved the architecture of the fibrillated OCNF network at the nanometer length scale, enabling comparison of the rheological properties of the OCNF-based gels under conditions where the network architecture is the same.
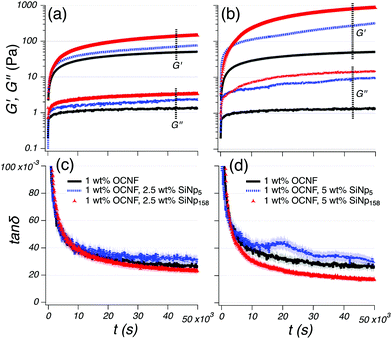
The equilibration of the rheological behaviour of the OCNF-based gels was evaluated after breakage (30 s at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 300 s−1) (Fig. 3). After the imposed breakage, both G′ and G′′ of the 1 wt% OCNF gel followed a rapid change (up to ca. 10 × 103 s) succeeded by a less pronounced evolution, indicating the dynamic nature of the OCNF network. Both filler-loaded gels showed higher values of G′ and G′′ compared to the pure OCNF gel, indicating an overall augmented toughness of the gels (Fig. 3a and b). Nevertheless, it must be noticed that the magnitude of G′ and G′′ does not necessarily describe the elastic/viscous-like balance of materials, but, for instance, its increase may be simply due to the increase in volume fraction upon filler addition. On the other hand, tan
= 300 s−1) (Fig. 3). After the imposed breakage, both G′ and G′′ of the 1 wt% OCNF gel followed a rapid change (up to ca. 10 × 103 s) succeeded by a less pronounced evolution, indicating the dynamic nature of the OCNF network. Both filler-loaded gels showed higher values of G′ and G′′ compared to the pure OCNF gel, indicating an overall augmented toughness of the gels (Fig. 3a and b). Nevertheless, it must be noticed that the magnitude of G′ and G′′ does not necessarily describe the elastic/viscous-like balance of materials, but, for instance, its increase may be simply due to the increase in volume fraction upon filler addition. On the other hand, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ better correlates with the elastic/viscous-like contributions of materials despite changes in volume fraction (Fig. 3c and d). The tan
δ better correlates with the elastic/viscous-like contributions of materials despite changes in volume fraction (Fig. 3c and d). The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of the 1 wt% OCNF gel containing 2.5 and 5 wt% SiNp5 displayed a very similar tan
δ of the 1 wt% OCNF gel containing 2.5 and 5 wt% SiNp5 displayed a very similar tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ profile to the 1 wt% OCNF gel up to 10 × 103 s, whilst, at longer times, it showed higher values, indicating that the addition of SiNp5 increases the viscous contribution of the gel without evident change of the elasticity. Contrarily, the gel containing 2.5 and 5 wt% of SiNp158 had a more pronounced decrease in tan
δ profile to the 1 wt% OCNF gel up to 10 × 103 s, whilst, at longer times, it showed higher values, indicating that the addition of SiNp5 increases the viscous contribution of the gel without evident change of the elasticity. Contrarily, the gel containing 2.5 and 5 wt% of SiNp158 had a more pronounced decrease in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ compared to the OCNF profile alone, indicating augmented elasticity of the gel.
δ compared to the OCNF profile alone, indicating augmented elasticity of the gel.
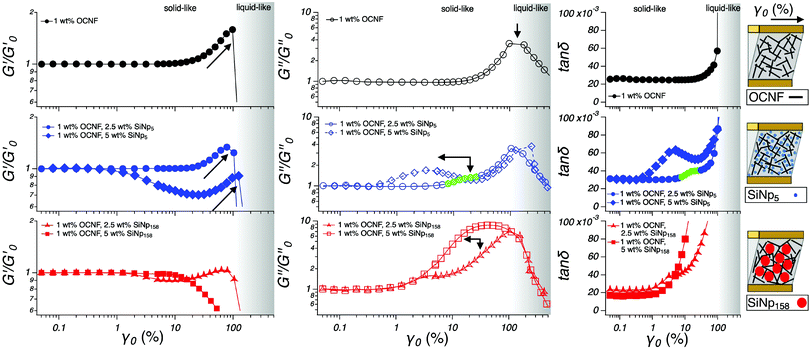
A strain sweep spacing from a small up to large deformation was employed to reveal how the filler size affects the OCNF network (Fig. 4). At small deformations, the 1 wt% OCNF gel showed a clear LVR, whilst at higher deformations, both G′ and G′′ showed a pronounced strain overshoot (signal increase followed by a decrease). Similar G′ and G′′ overshoots have been associated with the balance between breakage and regeneration of the network junctions.28 Specifically, G′ local maxima could arise by the increased connectivity between the fibrils occurring upon deformation, increasing the elastic contribution of the network. However, the presence of local maxima in G′′ would further indicate a co-occurring energy dissipation process, consequent to the network breakage. This is depicted by several network models as the balance between the formation and loss of the network junctions upon large deformations.29,30 The G′ and G′′ overshoots have been classified by Hyun et al. as a strong strain overshoot.28 The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ profile only displayed the upturn corresponding to the onset of yielding due to the higher G′′ overshoot compared to the G′ overshoot. When either 2.5 or 5 wt% SiNp5 was added into the OCNF gel, G′ overshoots were appreciable, suggesting that SiNp5 has little influence on the gel mechanics. On the other hand, the G′′ profile of the SiNp5 containing gel showed a similar overshoot as the pure OCNF gel although with an extra contribution appearing at lower values of γ0. The G′ overshoot for the OCNF gel containing 2.5 wt% SiNp158 was strongly smoothed out and was completely absent for the 5 wt% SiNp158 gel. The respective G′′ overshoots became remarkable, indicating a pronounced energy dissipation process upon deformation due to the loss of network junctions. The pronounced dissipative process occurring for the SiNp158 containing gel is well captured by tan
δ profile only displayed the upturn corresponding to the onset of yielding due to the higher G′′ overshoot compared to the G′ overshoot. When either 2.5 or 5 wt% SiNp5 was added into the OCNF gel, G′ overshoots were appreciable, suggesting that SiNp5 has little influence on the gel mechanics. On the other hand, the G′′ profile of the SiNp5 containing gel showed a similar overshoot as the pure OCNF gel although with an extra contribution appearing at lower values of γ0. The G′ overshoot for the OCNF gel containing 2.5 wt% SiNp158 was strongly smoothed out and was completely absent for the 5 wt% SiNp158 gel. The respective G′′ overshoots became remarkable, indicating a pronounced energy dissipation process upon deformation due to the loss of network junctions. The pronounced dissipative process occurring for the SiNp158 containing gel is well captured by tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, where the abrupt upturn occurs at lower values of γ0 compared to the OCNF gel. Differently, for the SiNp5, the overshoots are still visible in the tan
δ, where the abrupt upturn occurs at lower values of γ0 compared to the OCNF gel. Differently, for the SiNp5, the overshoots are still visible in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ profile due to the stronger G′′ overshoot compared to the G′ overshoot. Both fillers, SiNp5 and SiNp158, showed a shift of the G′′ overshoots towards lower values of γ0 at higher filler concentrations. This can be explained by the higher number of particles which would require lower deformation to cage, allowing an earlier onset of the dissipative energy. SiNp5 and SiNp158 belong, respectively, to a similar and a greater (ca. by a factor 5) length scale compared to the mesh size of the OCNF network.9 This information coupled with the herein described rheological measurements indicates that, when strong attractive interactions between the network and the filler could be ruled out (e.g. electrostatic attraction, hydrophobic interactions), the filler-to-mesh-size ratio is of great importance to induce specific rheological responses. Specifically, it is possible to attribute the unchanged elastic fingerprint (G′ profile) of the OCNF–SiNp5 gels to the possibility of the small filler particles to freely move within the aqueous phase of the OCNF network, only mildly affecting the breakage dynamics of the OCNF network upon oscillatory strain sweep. By contrast, the larger filler, SiNp158, would not have available free space, leading to completely different breakage dynamics where the G′ overshoot disappears and the dissipative contribution displayed by G′′ increases substantially (Fig. 4, schematics). Moreover, in agreement with the lack of strong OCNF–SiNp interactions, the addition of SiNp158 into the OCNF gel resulted in a transition from a strong strain overshoot (G′ and G′′ increase followed by a decrease) to a weak strain overshoot (only G′′ increase followed by a decrease) due to the weakening of the associative forces between the building blocks of the network, leading to a smoother structural breakage (yielding) upon deformation.28 The dynamic moduli, G′ and G′′, are calculated based on the assumption of the sinusoidal stress response of the material.31,32 Although G′ and G′′ provide a robust way to obtain structural information in the LVR, this assumption loses rigorous mathematical support in the NLVR, where the stress response deviates from being sinusoidal, as usually the case for large deformations.31,32
δ profile due to the stronger G′′ overshoot compared to the G′ overshoot. Both fillers, SiNp5 and SiNp158, showed a shift of the G′′ overshoots towards lower values of γ0 at higher filler concentrations. This can be explained by the higher number of particles which would require lower deformation to cage, allowing an earlier onset of the dissipative energy. SiNp5 and SiNp158 belong, respectively, to a similar and a greater (ca. by a factor 5) length scale compared to the mesh size of the OCNF network.9 This information coupled with the herein described rheological measurements indicates that, when strong attractive interactions between the network and the filler could be ruled out (e.g. electrostatic attraction, hydrophobic interactions), the filler-to-mesh-size ratio is of great importance to induce specific rheological responses. Specifically, it is possible to attribute the unchanged elastic fingerprint (G′ profile) of the OCNF–SiNp5 gels to the possibility of the small filler particles to freely move within the aqueous phase of the OCNF network, only mildly affecting the breakage dynamics of the OCNF network upon oscillatory strain sweep. By contrast, the larger filler, SiNp158, would not have available free space, leading to completely different breakage dynamics where the G′ overshoot disappears and the dissipative contribution displayed by G′′ increases substantially (Fig. 4, schematics). Moreover, in agreement with the lack of strong OCNF–SiNp interactions, the addition of SiNp158 into the OCNF gel resulted in a transition from a strong strain overshoot (G′ and G′′ increase followed by a decrease) to a weak strain overshoot (only G′′ increase followed by a decrease) due to the weakening of the associative forces between the building blocks of the network, leading to a smoother structural breakage (yielding) upon deformation.28 The dynamic moduli, G′ and G′′, are calculated based on the assumption of the sinusoidal stress response of the material.31,32 Although G′ and G′′ provide a robust way to obtain structural information in the LVR, this assumption loses rigorous mathematical support in the NLVR, where the stress response deviates from being sinusoidal, as usually the case for large deformations.31,32
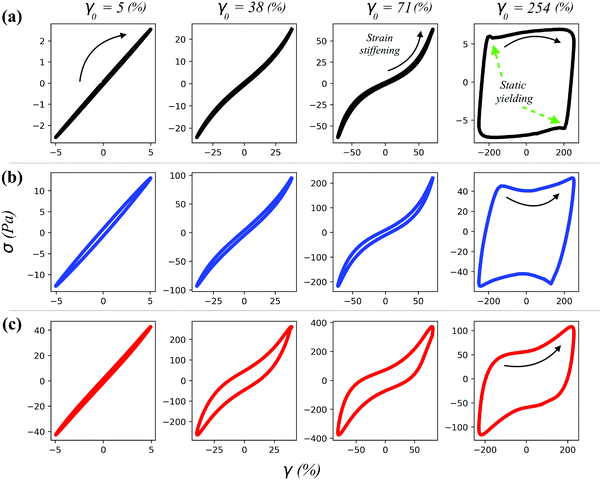
In the case of the herein described gels, the richest rheological behaviour lies in the large deformation range; hence, a waveform inspection was conducted via plotting the stress response (σ) as a function of the instantaneous intracycle strain (γ) (Fig. 5), commonly known as the elastic projection of Lissajous plot (for the viscous projection as σ vs. shear rate (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) see Fig. S3 (ESI†), whilst for the applied sinusoidal deformation as γ vs. time and the corresponding stress response as σ vs. time see Fig. S4, ESI†).31,32 At a low deformation (γ0 = 5%), the Lissajous plots of the gels showed a perfectly elliptical shape which encloses a little portion of area, indicating the dominant linear response and the dominant elastic behaviour of the material, respectively (Fig. 5). By contrast, at higher deformations (γ0 = 38 and 71%), the Lissajous plots acquired a more distorted shape, indicating the deviation of the stress signal from linearity. At the largest deformation (γ0 = 254%), the stress response was predominantly viscous and non-linear as indicated by the squared shape of the Lissajous plot. It is important to notice that this trend reflects that displayed by G′ and G′′, indicating that the physical interpretation of G′ and G′′ being proportional to the average energies stored and dissipated per cycle, respectively, is not violated. For γ0 values of 38 and 71%, the stress response, starting at σ = 0, followed a linear increase with the strain (elastic straining), after which a more pronounced stress increase was observed, indicating strain stiffening at greater intracycle deformations (γ0 = 71% in Fig. 5), which is in good relation to the G′ overshoot displayed in Fig. 4. Similar strain stiffening phenomena have been reported as ubiquitous in any network composed of semiflexible filamentous proteins33 although found difficult to mimic using commercially available polymers.34 The origin of strain stiffening in fractal aggregates formed by a diffusion-limited cluster aggregation process, as expected for the OCNF network herein reported, has been proposed to arise from the intrinsic stiffness of the cluster backbone.35 For γ0 = 38 and 71%, where the solid-like behaviour dominates (G′ > G′′), the shape of the Lissajous plots of the pure OCNF and the 5 wt% SiNp5 loaded gel showed similar features. In the presence of the 5 wt% SiNp158, the Lissajous plots displayed a more opened structure, indicating a pronounced energy dissipation (proportional to the area enclosed in the Lissajous plot in the σ vs. γ0 projection), and a milder intracycle strain stiffening, underpinning the reorganization of the fibrillar network occurring upon deformation. At higher deformations, γ0 = 254%, the dominating liquid-like behaviour (G′ < G′′) was underlined by the appearance of the stress shoulder associated with the static yielding prior to flow, where the static yielding is defined as the stress that needs to be overcome to make the material flow.31 At γ0 = 254%, the stress shoulder became broader for both filler loaded gels and, after the static yielding, displayed an abrupt stress decrease followed by an increase (indicated by the arrow). These similarities appeared solely in the Lissajous plots of the filler-loaded gels, suggesting that at higher deformations, after the static yield point, the flow properties are characterised by the filler. To better interpret the non-linear behaviour, we employed the framework of Rogers et al.,31 which decomposes the whole amplitude cycle into separate sequences of events. Although different approaches have been developed to analyse Lissajous plots, the one proposed by Rogers et al.31 better links rheological events to structural properties.36,37 In particular, we characterize the linear stress response starting at σ = 0 by the local cage modulus Gcage as follows:
) see Fig. S3 (ESI†), whilst for the applied sinusoidal deformation as γ vs. time and the corresponding stress response as σ vs. time see Fig. S4, ESI†).31,32 At a low deformation (γ0 = 5%), the Lissajous plots of the gels showed a perfectly elliptical shape which encloses a little portion of area, indicating the dominant linear response and the dominant elastic behaviour of the material, respectively (Fig. 5). By contrast, at higher deformations (γ0 = 38 and 71%), the Lissajous plots acquired a more distorted shape, indicating the deviation of the stress signal from linearity. At the largest deformation (γ0 = 254%), the stress response was predominantly viscous and non-linear as indicated by the squared shape of the Lissajous plot. It is important to notice that this trend reflects that displayed by G′ and G′′, indicating that the physical interpretation of G′ and G′′ being proportional to the average energies stored and dissipated per cycle, respectively, is not violated. For γ0 values of 38 and 71%, the stress response, starting at σ = 0, followed a linear increase with the strain (elastic straining), after which a more pronounced stress increase was observed, indicating strain stiffening at greater intracycle deformations (γ0 = 71% in Fig. 5), which is in good relation to the G′ overshoot displayed in Fig. 4. Similar strain stiffening phenomena have been reported as ubiquitous in any network composed of semiflexible filamentous proteins33 although found difficult to mimic using commercially available polymers.34 The origin of strain stiffening in fractal aggregates formed by a diffusion-limited cluster aggregation process, as expected for the OCNF network herein reported, has been proposed to arise from the intrinsic stiffness of the cluster backbone.35 For γ0 = 38 and 71%, where the solid-like behaviour dominates (G′ > G′′), the shape of the Lissajous plots of the pure OCNF and the 5 wt% SiNp5 loaded gel showed similar features. In the presence of the 5 wt% SiNp158, the Lissajous plots displayed a more opened structure, indicating a pronounced energy dissipation (proportional to the area enclosed in the Lissajous plot in the σ vs. γ0 projection), and a milder intracycle strain stiffening, underpinning the reorganization of the fibrillar network occurring upon deformation. At higher deformations, γ0 = 254%, the dominating liquid-like behaviour (G′ < G′′) was underlined by the appearance of the stress shoulder associated with the static yielding prior to flow, where the static yielding is defined as the stress that needs to be overcome to make the material flow.31 At γ0 = 254%, the stress shoulder became broader for both filler loaded gels and, after the static yielding, displayed an abrupt stress decrease followed by an increase (indicated by the arrow). These similarities appeared solely in the Lissajous plots of the filler-loaded gels, suggesting that at higher deformations, after the static yield point, the flow properties are characterised by the filler. To better interpret the non-linear behaviour, we employed the framework of Rogers et al.,31 which decomposes the whole amplitude cycle into separate sequences of events. Although different approaches have been developed to analyse Lissajous plots, the one proposed by Rogers et al.31 better links rheological events to structural properties.36,37 In particular, we characterize the linear stress response starting at σ = 0 by the local cage modulus Gcage as follows:
| Gcage = dσ/dγ|σ=0 |
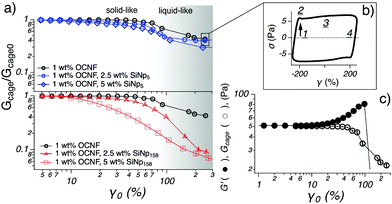 | ||
| Fig. 6 (a) Gcage normalised by the respective modulus in the LVR (Gcage0 = G0′) as a function of strain (γ0) for the OCNF-based gels. The 1 wt% OCNF gel is shown in both graphs as a reference. The darkening of the background refers to the transition from solid-like (G′ > G′′) to liquid-like (G′ < G′′) for the 1 wt% OCNF gel and is drawn to guide the eye. (b) The Lissajous plot is drawn to depict the sequence of events which soft materials undergo upon LAOS: straining (1), static yielding (2), flowing (3) and reformation (4), as suggested by Rogers et al.31 (c) Strain sweep for the 1 wt% OCNF gel showing non-normalised G′ and Gcage as a function of γ0. The displayed data are obtained from a set of duplicate samples; the respective error is within the size of the symbol for γ0 < 100 (%). | ||
4 Conclusions
In this study, we investigated the effect of the filler size on the structural and mechanical properties of an attractive fibrillated network composed of OCNF, under conditions where strong filler–filler and filler–fibril interactions are absent. The two fillers, SiNp5 and SiNp158, were chosen to be of dimensions similar to and greater than the gel network mesh size, respectively. Small angle neutron scattering revealed that the OCNF network preserved its nanoscale architecture in the presence of both fillers, whilst oscillatory shear rheology captured clear rheological differences. Large amplitude oscillatory shear (LAOS) displayed the richest rheological behaviour and allowed to access the impact of the filler-to-mesh-size ratio on the yielding behaviour of the hydrogel. Our findings indicate that the presence of the smaller filler, SiNp5, in the OCNF network maintains the mechanics of the network almost unvaried. This phenomenon is associated with the ability of SiNp5 to fit in the mesh size of the gel network without altering the dynamics of the network upon large amplitude oscillatory shear. In contrast, the presence of the larger filler, SiNp158, resulted in a gradual structural breakage and a low network recoverability after the breakage induced by large deformations, suggesting that SiNp158 could actively dislodge the junctions of the network. The fundamental understanding provided in this study has implications for industrially relevant formulations, where the efficacy of additives in primary matrixes is of main importance towards tunability of specific structural and mechanical properties on demand.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors thank the EPRSC for funding this project (grant number EP/N033310/1). V. Calabrese thanks the University of Bath for funding his PhD studentship. The authors thank Prof. Roger Rothon for the generous donation of SiNp5 from Geo40™. The authors thank the Institut Laue Langevin for the provision of neutron beam time on D22 instrument provided under the Easy Access System (the data set related to this beam time is archived at DOI: 10.5291/ILL-DATA.EASY-477). Data supporting this work are freely accessible in the Bath Research Data Archive System at DOI: 10.15125/BATH-00762.Notes and references
- K. J. De France, T. Hoare and E. D. Cranston, Chem. Mater., 2017, 29, 4609–4631 CrossRef CAS.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- T. Saito, Y. Nishiyama, J. L. Putaux, M. Vignon and A. Isogai, Biomacromolecules, 2006, 7, 1687–1691 CrossRef CAS PubMed.
- Y. Habibi, H. Chanzy and M. R. Vignon, Cellulose, 2006, 13, 679–687 CrossRef CAS.
- T. Saito, T. Uematsu, S. Kimura, T. Enomae and A. Isogai, Soft Matter, 2011, 7, 8804–8809 RSC.
- J. Y. Park, C. W. Park, S. Y. Han, G. J. Kwon, N. H. Kim and S. H. Lee, Polymers, 2019, 11, 414 CrossRef PubMed.
- H. Fukuzumi, R. Tanaka, T. Saito and A. Isogai, Cellulose, 2014, 21, 1553–1559 CrossRef CAS.
- F. Fneich, J. Ville, B. Seantier and T. Aubry, Carbohydr. Polym., 2019, 211, 315–321 CrossRef CAS PubMed.
- J. Schmitt, V. Calabrese, M. A. Da Silva, S. Lindhoud, V. Alfredsson, J. L. Scott and K. J. Edler, Phys. Chem. Chem. Phys., 2018, 20, 16012–16020 RSC.
- L. Mendoza, W. Batchelor, R. F. Tabor and G. Garnier, J. Colloid Interface Sci., 2018, 509, 39–46 CrossRef CAS PubMed.
- R. J. Crawford, K. J. Edler, S. Lindhoud, J. L. Scott and G. Unali, Green Chem., 2012, 14, 300–303 RSC.
- B. L. Tardy, S. Yokota, M. Ago, W. Xiang, T. Kondo, R. Bordes and O. J. Rojas, Curr. Opin. Colloid Interface Sci., 2017, 29, 57–67 CrossRef CAS.
- K. Chen, Y. Xu, S. Rana, O. R. Miranda, P. L. Dubin, V. M. Rotello, L. Sun and X. Guo, Biomacromolecules, 2011, 12, 2552–2561 CrossRef CAS PubMed.
- H. Oguzlu and Y. Boluk, Cellulose, 2017, 24, 131–146 CrossRef CAS.
- E. Dickinson and J. Chen, J. Dispersion Sci. Technol., 1999, 20, 197–213 CrossRef CAS.
- T. Vliet, Colloid Polym. Sci., 1988, 266, 518–524 CrossRef.
- A. J. Gravelle, S. Barbut and A. G. Marangoni, RSC Adv., 2015, 5, 60723–60735 RSC.
- T. Gillece, R. L. Mcmullen, H. Fares, L. Senak, S. Ozkan and L. Foltis, J. Cosmet. Sci., 2016, 67, 121–159 Search PubMed.
- V. Calabrese, J. C. Muñoz-García, J. Schmitt, M. A. da Silva, J. L. Scott, J. Angulo, Y. Z. Khimyak and K. J. Edler, J. Colloid Interface Sci., 2019, 535, 205–213 CrossRef CAS PubMed.
- T. Saito and A. Isogai, Biomacromolecules, 2004, 5, 1983–1989 CrossRef CAS PubMed.
- I. Grillo, Soft Matter Characterization, Springer, Netherlands, Dordrecht, 2008, pp. 27–30 Search PubMed.
- M. Adamo, A. S. Poulos, R. M. Miller, C. G. Lopez, A. Martel, L. Porcar and J. T. Cabral, Lab Chip, 2017, 17, 1559–1569 RSC.
- J. Ilavsky and P. R. Jemian, J. Appl. Crystallogr., 2009, 42, 347–353 CrossRef CAS.
- S. R. Kline, J. Appl. Crystallogr., 2006, 39, 895–900 CrossRef CAS.
- K. M. Weigandt, D. C. Pozzo and L. Porcar, Soft Matter, 2009, 5, 4321 RSC.
- M. Kobayashi, F. Juillerat, P. Galletto, P. Bowen and M. Borkovec, Langmuir, 2005, 21, 5761–5769 CrossRef CAS PubMed.
- M. A. da Silva, V. Calabrese, J. Schmitt, D. Celebi, J. L. Scott and K. J. Edler, Soft Matter, 2018, 14, 9243–9249 RSC.
- K. Hyun, S. H. Kim, K. H. Ahn and S. J. Lee, J. Non-Newtonian Fluid Mech., 2002, 107, 51–65 CrossRef CAS.
- K. H. Ahn and K. Osaki, J. Non-Newtonian Fluid Mech., 1994, 55, 215–227 CrossRef CAS.
- H. G. Sim, K. H. Ahn and S. J. Lee, J. Non-Newtonian Fluid Mech., 2003, 112, 237–250 CrossRef CAS.
- S. A. Rogers, B. M. Erwin, D. Vlassopoulos and M. Cloitre, J. Rheol., 2011, 55, 435–458 CrossRef CAS.
- K. Hyun, M. Wilhelm, C. O. Klein, K. S. Cho, J. G. Nam, K. H. Ahn, S. J. Lee, R. H. Ewoldt and G. H. McKinley, Prog. Polym. Sci., 2011, 36, 1697–1753 CrossRef CAS.
- C. Storm, J. J. Pastore, F. C. MacKintosh, T. C. Lubensky and P. A. Janmey, Nature, 2005, 435, 191–194 CrossRef CAS PubMed.
- R. H. Ewoldt, C. Clasen, A. E. Hosoi and G. H. McKinley, Soft Matter, 2007, 3, 634–643 RSC.
- T. Gisler, R. C. Ball and D. A. Weitz, Phys. Rev. Lett., 1999, 82, 1064–1067 CrossRef CAS.
- J. C.-W. Lee, L. Porcar and S. A. Rogers, Polymers, 2019, 11, 1189 CrossRef PubMed.
- C.-W. Lee and S. A. Rogers, Korea-Aust. Rheol. J., 2017, 29, 269–279 CrossRef.
- K. van der Vaart, Y. Rahmani, R. Zargar, Z. Hu, D. Bonn and P. Schall, J. Rheol., 2013, 57, 1195–1209 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Contrast match determination for SiNp, frequency sweeps, viscous projection of Lissajous plots, sinusoidal deformation and non-normalised data. See DOI: 10.1039/c9sm02175b |
| ‡ Current address: Micro/Bio/Nanofluidics Unit, Okinawa Institute of Science and Technology, Onna, Okinawa 904-0495, Japan. |
| This journal is © The Royal Society of Chemistry 2020 |