Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogen via reforming aqueous ammonia and biomethane co-products of wastewater treatment: environmental and economic sustainability
Oliver
Grasham
*a,
Valerie
Dupont
a,
Timothy
Cockerill
a,
Miller Alonso
Camargo-Valero
 b and
Martyn V.
Twigg
c
b and
Martyn V.
Twigg
c
aSchool of Chemical and Process Engineering, The University of Leeds, LS2 9JT, UK. E-mail: O.R.Grasham@leeds.ac.uk
bSchool of Civil Engineering, The University of Leeds, LS2 9JT, UK
cTwigg Scientific and Technical Ltd, Caxton, Cambridge, CB23 3PQ, UK
First published on 2nd October 2020
Abstract
Green H2 is increasingly viewed as a key energy carrier for the fight against climate change. Wastewater treatment plants (WWTPs) have the unique potential to be centres of renewable H2 generation with the growing availability of two attractive feedstocks: biomethane and ammonia. An innovative and novel method of ammonia recovery from digestate liquor followed by a state-of-the-art H2 production process named NWaste2H2 is demonstrated for a case-study WWTP. The recovered ammonia is used alongside biomethane for H2 production and its diversion from conventional biological treatment has two other crucial benefits, with reductions in both associated electricity demand and emissions of nitrous oxide, an extremely potent greenhouse gas. Process modelling, supported by extensive experiments in a packed-bed reactor at bench-scale, demonstrate the prized capability of simultaneously performing steam methane reforming and ammonia decomposition to generate a H2-rich syngas with yields close to equilibrium values. Greenhouse gas emission abatement from the replacement of diesel buses and reduced N2O emissions from biological treatment could save up to 17.2 kg CO2 equivalent (CO2e) per year for each person served by the WWTP. An in-depth economic study illustrates the ability to achieve a positive net present value with a 10% discount factor as early as 5.8 years when the H2 is prepared and sold to power fuel cell electric buses.
1 Introduction
Interest in the use of H2 as an energy vector has made a significant resurgence in recent years. According to many in industry, government and academia, green H2 has the potential to play an important part in the global transition to carbon-free economies.1–4 There are numerous reasons, such as its ability to be used in existing infrastructure, carbon and pollution free emissions at use, historical utilisation in industry and its potential to penetrate all energy sectors (heat, power, transport and storage).5 However, economically appealing methods of H2 production from biogenic sources have been hard to come by,6 something that may change with findings presented in this article.Green H2 can be produced by electrolysis of water using renewable electricity, steam methane/natural gas reforming (SMR) with carbon capture and storage (CCS), dark fermentation and biomass reforming and gasification.7 H2 generation via electrolysis from renewables and SMR with CCS are being touted as the most promising technology routes for significant energy market penetration.5 However, other generation methods should not be disregarded. One biogenic feedstock gaining interest is biogas from wastewater treatment plants (WWTPs), which can be steam reformed to produce H2.8–10 In the UK alone, WWTPs produce enough biogas to generate >1 TW helec each year,11 proving it as a widely available potential H2 source.
WWTPs also feature a hidden, largely unutilised hydrogen carrier in the form of ammonia. The liquor fraction of anaerobic digestate, which contains a considerable quantity of ammonia, is ordinarily recycled back into the treatment process. Air stripping-absorption is the most common method of ammonia recovery, with multiple companies providing commercially available products that are typically used to produce fertilisers.12 Scarce examples of process modelling for ammonia recovery for generation of fertilisers exist, with Errico et al.13 providing an almost stand-alone case. As of August 2020, the market value of fertiliser in the UK is at £0.2 per kg ammonium nitrate (£0.58 per kg N).14 This makes it difficult for the recovery of ammonia from digestate liquor to be profitable. As such, this paper aims to highlight an alternative use for aqueous ammonia recovered from digestate which could be more attractive to wastewater treatment plant operators. At a market value of £4.50 per kg H2 a conversion from ammonia could have a value of up to £0.81 per kg N.
If recovered effectively in a highly pure stream (as opposed to a fertiliser), it could provide a top up of H2 alongside that produced via the reforming of biogas/biomethane. Yet there is the potential to recover this ammonia and a potential use of it is to thermally crack it to form H2 and nitrogen (eqn (11)). This was in some capacity demonstrated in a paper by Grasham et al.,15 where the recovery of ammonia from digestate liquor at a case study WWTP was modelled along with its consequent use with bio-methane in a solid-oxide fuel cell for the generation of heat and power. Here, ammonia cracking and SMR occur within the SOFC to facilitate the cogeneration of heat and power.
A significant advantage of diverting ammonia from conventional treatment is the positive impact on a WWTP's energy consumption and emissions of greenhouse gases (GHGs). The release of ammoniacal and nitrate nitrogen at WWTPs is restricted under EU law16,17 due to the damaging consequences of emitting ammonia and nitrates into aquatic ecosystems. The most popular method of achieving NH3 conversion to N2 is via biological nitrification and denitrification in a multi-stage activated sludge process (ASP). Unfortunately, the aerobic bacteria involved in nitrification require considerable amounts of oxygen to be pumped into tanks, increasing the WWTP's electricity consumption. Garrido et al.18 calculated that 4.57 kW h of electrical energy are required for every kg of oxidised nitrogen.
A further noteworthy issue with nitrification–denitrification systems is the generation of nitrous oxide (N2O) as an intermediary and side-product.19 N2O is a particularly potent GHG with a global warming potential (GWP) 298 times that of CO2 over a 100 year period. The UK Water Industry Research Ltd suggest an emission factor of 0.002 kg of N in the form of N2O (‘N2O–N’) for every kg of nitrogen in the WWTP load.20 Moreover, N2O emissions from wastewater treatment are notoriously variable. For example, Kampschreur et al.19 found N2O emissions of 0–14.6% of the nitrogen load from a review of literature on emissions from full scale operational facilities. Meanwhile, Parravicini et al.21 have developed a regression model for estimating N2O emissions based on the destruction of nitrogen during conventional treatment. Regardless of the exact emission factor, diversion of ammonia found in digestate liquor from activated sludge treatment could significantly improve a facility's energy consumption and carbon footprint.
H2 fuel cell electric buses have been identified as a notably attractive option to decrease carbon emissions from transport, whilst also improving urban air quality.22,23 A recent report by the Hydrogen Council found that fuel cell buses were the most competitive way to decarbonise medium-long range bus travel.24 According to the IEA, by the end of 2019 there were around 4600 fuel-cell electric buses globally.25 Ten of these fuel cell buses are currently in use in Aberdeen, UK and have been praised for their influence in the city's recent emission reductions.26 Hatch et al.27 performed a techno-economic analysis of H2 production scenarios at WWTPs as a fuel for bus transport, concluding that steam methane reforming (SMR) of bio-methane could be a cost-competitive option for decarbonising a fleet of buses. Furthermore, it showed that SMR was the most commercially attractive technology option when compared to alternatives including molten carbonate fuel cells (MCFCs) and wood gasification. Given the current GHG emissions from diesel buses, a conversion to green H2 could save almost 1.3 kg CO2e per km.28
This work details the feasibility of innovatively combining ammonia recovery and H2 production system at wastewater treatment plants in a process we named NWaste2H2, with the use of generated H2 as a bus fleet transportation fuel. The pioneering system, which uses both ammonia catalytic decomposition and methane steam reforming to produce H2 simultaneously, has been modelled and optimised using Aspen Plus® V.10.29 A techno-economic study has been carried out to determine the financial feasibility and attractiveness of implementing the process at an operational WWTP in the UK. To the authors' knowledge this is the first instance of reporting the cumulative benefits of greenhouse gas emissions savings during green H2 production by using a combination of both biogas and bio-ammonia feedstock, with a sizeable part of the savings being achieved by suppression of nitrous oxide emission from the main WWTP process. It is suggested that wide-spread process implementation, could provide an important contribution to global H2 infrastructure with its economic competitiveness, whilst also reducing WWTP GHG emissions. The tangible viability of generating H2 in the proposed format has been analysed via a unique experimental procedure combining steam methane reforming and ammonia decomposition carried out in a packed-bed reactor over a catalyst.
2 Methods for process analysis
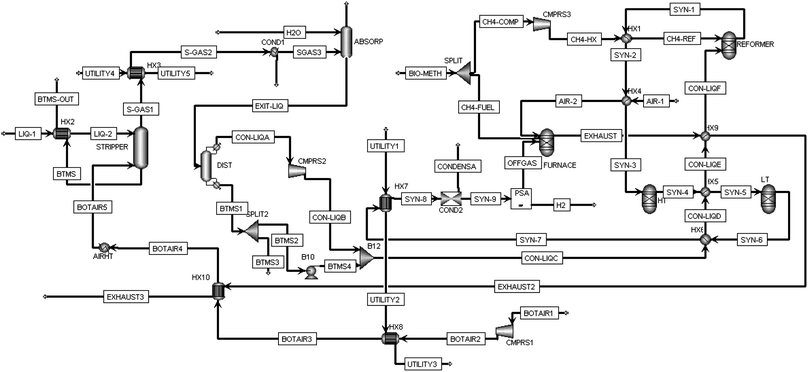
A chemical process model was developed in Aspen Plus® V.10 (ref. 29) and sensitivity analysis was carried out to provide an energy efficient method of NH3 recovery from a low concentration ammonia solution representative of digestate liquor. This was combined with a process simulation for H2 generation via a combination of the catalytic processes of steam bio-methane reforming, water–gas-shift and bio-ammonia decomposition. A simplified process flow diagram of these steps can be found in Fig. 1 with a more detailed version in Fig. 3. The removal of CO2 and H2S from the biogas has not been modelled in Aspen Plus® but included in economic analysis as a high-pressure water scrubbing system. The following assumptions were also utilised throughout: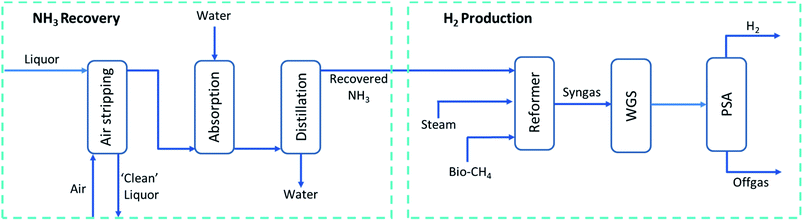 | ||
| Fig. 1 Simplified process flow diagram (PFD) of process modelled using Aspen Plus®. Full PFD found in Fig. 3. | ||
• Ambient conditions set at 1 bar, 23 °C.
• Air composition assumed 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 split of N2
21 split of N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 only.
O2 only.
Biomethane and digestate liquor flows and liquor ammonia concentrations are based on data from a real WWTP serving a population equivalent to 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people in the UK. Ammonia concentration of the digestate liquor was determined via analysis of 11 samples from the reference WWTP between October 2014 and May 2016 by members of the BioResource Systems Research Group at the University of Leeds. The 4500 N Standard Method as reported in ref. 30 was used for determination, detailing an average concentration of 1.5 g L−1.
000 people in the UK. Ammonia concentration of the digestate liquor was determined via analysis of 11 samples from the reference WWTP between October 2014 and May 2016 by members of the BioResource Systems Research Group at the University of Leeds. The 4500 N Standard Method as reported in ref. 30 was used for determination, detailing an average concentration of 1.5 g L−1.
The WWTP's current energy usage stands at 60 MW helec per day and generates 40 MW helec per day via conventional cogeneration technology fuelled with biogas, thus operates with a net energetic deficit of 20 MW helec each day. Biomethane production of 8229 kg per day was back calculated from the electricity generation based on a 35% electrical efficiency of the plant's combined heat and power CHP units (LHV basis). The WWTP can be considered ‘large’ in a UK context, producing 30% more power than the industry mean. Digestate liquor production of 661 m3 per day was asserted from an equivalence of 0.63% of total wastewater inflow, interpreted from,31 which stands at a daily average of 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day.
000 m3 per day.
N2O emission savings have been estimated by calculating an N2O emission factor based on the regression model developed by Parravicini et al.21 which estimates N2O emissions based on the % removal of total nitrogen (TN) during treatment equation:
| y = −0.049x + 4.553 | (1) |
The GHG emission implications of process implementation were determined viaeqn (2), where GHGtotal are the total savings GHGNH3,diverted are the savings facilitated by the diversion of ammonia from conventional biological treatment, GHGbus,abatement and GHGgrid,NG are the abated emissions from converting diesel-buses to H2, GHGgrid,elec are the emissions arising from the increased use of grid electricity and heat that would other-wise have been met by the plant's CHP system, all in units of CO2e, The grid-supplied power and heat have carbon intensities of 107 and 210 g CO2 per kW h respectively.34,35
| GHGtotal = GHGNH3,diverted + GHGbus,abatement – GHGgrid,elec – GHGgrid,NG | (2) |
3 Economic analysis methodology
The economic model developed in this work uses the aforementioned reference wastewater treatment plant as a case study for process implementation. The assumption was made that the CHP units have already returned their investment – making the facility open to alternative processing routes for the biogas generated. A net present value (NPV) analysis has been carried out to determine the financial robustness of process implementation (economic sustainability). NPV takes into account the time value of money, where the value of a sum of money is worth more in the present than the same sum of money will be worth in the future. Eqn (3) displays the calculation used for NPV: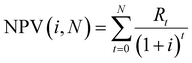 | (3) |
| RPI (%) | 2.87 |
| H2 fuel price (£) | 4.50 |
| RHI price (£ per kW h) | 0.0116 |
| Industry electricity price (£ per kW helec) | 0.1055 |
| Industry heat price (£ per kW hth) | 0.0233 |
| Scaling factor | 0.6 |
| Discount rate (%) | 10 |
| Annual maintenance (3%) | 3 |
| Large-scale replacement costs after 5, 10, 15 years (% of CAPEX) | 15, 50, 15 |
| Catalyst cost (£ per kg H2) | 0.15 |
| RTFO value (2018) | 0.15 |
| Employees/shift | 3.8 |
| Shifts/day | 3 |
| Personnel for 40 h week | 17.2 |
| Average salary (£) | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| Salary interest (%) | 2.99 |
Most equipment and installation costs have been determined using Aspen Plus® Economic Analyzer (APEA).36 The total installed costs which comprise the total CAPEX include: above ground piping, poling, concrete, instrumentation underground/above ground electrics, grout and labour costs. The National Renewable Energy Laboratory (NREL) have developed an economic model for a H2 production system37 with the inclusion of a H2 refuelling station. The NREL model was utilised to determine the total installation costs of units where APEA could not be used accurately, and an exchange rate of £0.78/$ has been used throughout. The calculation displayed in eqn (4), known as the six-tenths factor rule, was used to estimate the required adjustment in costs according to the scale difference with the NREL model.
| Cost of equipment a = cost of equipment bX0.6 | (4) |
In eqn (4), cost of equipment a and b are the unknown and known unit cost respectively, X is the multiplication factor for the known capacity difference between unit a and unit b (a/b), 0.6 represent the ‘scaling factor’ often used to approximate scaling costs. This method of cost estimation was used for the primary reformer, the two water–gas shift (‘WGS’) reactors, the pressure swing adsorber unit (PSA) and the pre-PSA condenser. The NREL economic model also includes a compression storage and dispensing (CSD) part of the plant which is hypothesised to be for the use of H2 powered buses. As such, the associated CSD costs detailed in the NREL model have been scaled for use in this body of work. The CSD system consists of the compression of generated H2 to 350 bar, ready for dispensing to H2-fuelled buses. The costing of the stripping gas pre-heater (AIRHT) has been estimated using the cost curve for a stainless steel direct fired heater as displayed in Peters et al. (pg. 692)38 and adjusted for inflation via long term UK retail price index (RPI). RPI has also been used as the figure for year-on-year interest rate.
Catalyst costs have been proposed at $0.19 per kg of H2 as detailed in Kaiwen et al.39 for nickel-based catalyst. The cost target of renewable H2 for transport has been stipulated by the European Union at £4.50 (€5) per kg, equivalent to the value discussed by Reuter et al.40 whereby H2 would be competitive with diesel-based bus transportation. This is further backed by the Hydrogen Council who state that H2 could competitively supply 30% of all transport with a price at the pump of $6 per kg (£4.60 per kg).24 Expenditure on heat has been calculated with a cost of 2.33 p per kW h as detailed in the BEIS report on gas and electricity prices in the UK non-domestic sector.41 The required expenditure for heat has been determined using the requirements of blocks AIRHT and DIST (displayed in Fig. 3) with a thermal transfer efficiency of 80%. This is required as the heat ready for export from the process is not of a high enough quality to meet the requirements of AIRHT and DIST. The electricity price has been set as the average for a ‘large’ power consumer in the UK at £10.58 pence per kW h.41 The expenditure on electricity has been valued via the power consumption of each compressor and pump simulated in Aspen Plus® and the power requirement of CSD calculated from the NREL economic model. Replacement costs have been inferred from the aforementioned NREL economic model37 whereby after five and fifteen years replacement costs equate to 15% of the initial capital investment and 50% after 10 years.
Cost of labour was calculated with the determination of required operators via data obtained by Alkhayat & Gerrard and described in Turton et al. as eqn (5).42,43 The calculation can be found in eqn (5) where, NOL is the number of operators per shift, PP is the number of processing steps involving the handling of particulate solids and Nnp is the number of non-particulate processing steps.
| NOL = (6.29 + 31.7PP2 + 0.23Nnp)0.5 | (5) |
The renewable transport fuel obligation (RTFO) is the UK's chief policy for reducing GHG emissions in the transport sector. RTFO certificates are granted to producers of low carbon/renewable fuel which can then be traded in an open market to organisations that have not met their certificate obligation. Renewable H2 is awarded 4.58 certificates per kg and a conservative market value of 15 p per certificate has been assigned in this study.44
Sensitivity analysis has been carried out to indicate which factors will play an important role in the overall feasibility of potential process integration. Figures for the H2 selling price, overall annual expense, initial capital investment and catalyst cost have been altered by ±15% to demonstrate the possible impact on the systems overall profitability in NPV terms. This sensitivity analysis also provides an indication of the security of the system's potential profitability.
4 Experimental methods
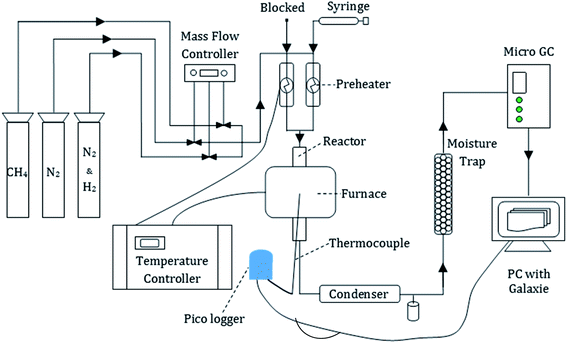
The primary reformer developed in the process model described in this article provides the platform for both ammonia decomposition and steam methane reforming. To the authors' knowledge this simultaneous method of H2 generation has not been tested in a packed-bed reactor using commercial grade catalysts. Therefore, the following experiments have been carried out to demonstrate the capability of ‘co-reforming’ methane and ammonia accordingly. Direct use of experimental data in the process model would be improper due to the disparity in thermodynamic conditions, notably that the reformer presented in the process model is running at a pressure of 25 bar compared to 1 atm experimentally. Nevertheless, by comparing the results to equilibrium equivalents it is possible to infer the validity of the data presented in the process model. Meanwhile experimental carbon deposition analysis was performed to highlight potential associated operational difficulties unidentified during equilibrium-based simulations.Fig. 2 illustrates a schematic of the bench-scale rig used for the combined ammonia decomposition and steam methane reforming experiments. Three forms of compressed gases were connected to the rig: CH4, N2 and a 5 vol% H2/95 vol% N2 mix. The flow of the gases was controlled by MKS (US) mass flow controllers. The CH4 flow represents biomethane from the WWTP, the N2 was used as a carrier gas and the H2/N2 mix was used to reduce the catalyst from its original oxidised nickel state to the catalytically active metallic Ni. The gases were passed through electrical pre-heaters before entrance into the reactor. Liquid feedstock (de-ionised water and/or aqueous ammonia solution) was supplied to the system via a 20 ml BD Plastipak syringe with a stainless steel Luer lock needle via a New Era (US) Pump System Inc (model NE-1000). The ammonia solution used was a 25 wt% ammonia solution from Fischer Scientific (US). The liquid feed passed through a preheater set at 150 °C to vaporise before entrance to the reactor. Heating tape was used to aid the maintenance of temperature between the base of the preheaters and the reactor. Much of the rig was insulated to prevent thermal losses as much as possible.
The reactor was made of a 316 stainless steel tube with an internal diameter of 9.8 mm and positioned inside an electric tube furnace (Elite Thermal Systems TSV10/20/85) which provided the resistive heating to reach the desired reactor temperature. The internal temperature was monitored via a thermocouple connected to a pico-logger console in communication with a PC. The thermocouple reached roughly half way up the tube and provided a support in which a bed of 4 μm mean diameter quartz wool sat. The quartz wool, in turn, provided a semi-permeable and non-reactive platform in which the powder catalyst could sit. A commercial grade 18 wt% NiO/α-Al2O3 catalyst from Twigg Scientific & Technical Ltd (UK) was crushed and sieved to a diameter between 150–250 μm for use in each experiment.
The outlet from the reactor was fed into a stainless steel condenser which cooled the product gas using a counter-flow of (30 vol%) ethylene glycol/(70 vol%) water cooling liquid at −2 °C. A stainless steel condensate trap was located after the condenser and could be detached in order to remove, store and later analyse any condensate generated. Downstream of the condenser, a stainless steel moisture trap, filled with silica gel beads, facilitated the removal of all moisture from the syngas to ensure only a dry gas entered the micro gas chromatograph (GC).
The Varian (US) CP 4900 micro GC provided compositional analysis of the dry product gas from the reactor. It contained two thermal conductivity detectors (TCD) and two columns. The first was a Molecular Sieve 5A plot column and was calibrated to detect the following gases: H2, O2, N2, CH4 and CO. The second was a Pora Plot Q column which was calibrated for measurement of CO2, CH4, C2H6, C2H4, C3H8 and C3H6 gas concentrations. Argon was used as its carrier for both columns in the Varian micro GC module.
Carbon deposition on the catalyst was determined using CHN analysis Thermo Scientific (US) Flash 2000 Elemental Analyzer. The ammoniacal nitrogen remaining in the condensate was determined using a Hach (US) AP3900 Laboratory Robot with Hach LCK 303 ammonium calorimetry cuvettes. It was found that over time the ammonia contained in the aqueous solution volatilised during sample preparation. This meant that the intended S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios were adjusted for actual S
C ratios were adjusted for actual S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C achieved in the reactor after analysis for comparison with equilibrium modelling. Equilibrium modelling was carried out using an RGibbs reactor in Aspen Plus® with identical inlet flows to the experiments so that comparisons between the two datasets could be made.
C achieved in the reactor after analysis for comparison with equilibrium modelling. Equilibrium modelling was carried out using an RGibbs reactor in Aspen Plus® with identical inlet flows to the experiments so that comparisons between the two datasets could be made.
N2 was used as the carrier gas through the rig but was also formed during the decomposition of ammonia. Ordinarily the known quantity of N2 outlet would be used as a reference to calculate the molar flows of the other gases used in the syngas. However, without knowledge of the extent of ammonia decomposition occurring, an alternative method of product flow analysis was necessary. Therefore, a ‘contribution analysis’ approach was taken whereby speculative amounts of ammonia decomposition were considered until the composition of the syngas matched that of the micro-GC output.
This was performed viaeqn (6)–(8). Eqn (6) describes via a N elemental balance, how the molar flow of any specie x from the reactor (ṅx(out)) can be calculated using the known molar flows of N2 into the reactor (ṅN2(in)), the N2 generated from ammonia decomposition, the mole fraction of N2 (yN2(out)) and the mole fraction (yx(out)) of specie x measured in the product gas. Potential ammonia conversion via decomposition was iteratively altered between 5 and 100% to provide hypothetical specie molar flows through eqn (6). Stoichiometry dictates that for each mole of CO generated (ṅCO) via SMR (reaction (9)) there would be three moles of H2. Similarly for every mole of CO2 generated (ṅCO2) via WGS (reaction (10)) there are 4 moles of H2. This provided the basis for eqn (7) which describes the production of H2 from the combined SMR and WGS reactions (ṅH2(contribution)). Eqn (7) was performed for runs with and without ammonia present, as it enables calculation of the difference in H2 flows from the reactor via SMR and WGS (ΔṅH2(contribution)). When this is taken from the measured difference in H2 flows from the reactor (ΔṅH2(out)) it was then possible to calculate the H2 provided solely by ammonia decomposition (ṅH2(NH3decomp)). During ammonia decomposition (reaction 11), for every mole of N2 generated, 3 moles of H2 are produced. As such, the true ṅH2(NH3decomp) was determined by iteration from the ammonia decomposition sensitivity analysis when it equalled three times that of ΔṅN2(out). True ΔṅH2(NH3decomp) value was then used to determine the percent ammonia conversion reported in the results.
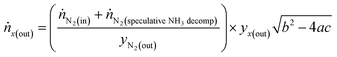 | (6) |
| ṅH2(contribution) = 3ṅCO + 4ṅCO2 | (7) |
| ṅH2(NH3decomp) = ΔṅH2(out) − ΔṅH2(contribution) | (8) |
Runs with and without ammonia were completed at temperatures 700 °C, 750 °C and 800 °C and at S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios close to S
C ratios close to S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios approximating the intended 2, 3 and 4 (precision adjusted afterwards). A constant weight hourly space velocity (WHSV = total mass flow rate divided by reactor bed mass load) of 2 h−1 was used in each experiment. The gas hourly space velocity (GHSV = total volumetric flow rate dived by volume of catalyst bed) varied between 4014 and 4234 h−1. Total experimental run time averaged at 3 hours which generated 50 data points from the micro-GC, providing enough time for robust analysis of the mean syngas composition and the extent of carbon deposition. Experimental feed compositions can be found in Table 7. Each condition was tested in duplicate and the runs with the least variability used for analysis.
C ratios approximating the intended 2, 3 and 4 (precision adjusted afterwards). A constant weight hourly space velocity (WHSV = total mass flow rate divided by reactor bed mass load) of 2 h−1 was used in each experiment. The gas hourly space velocity (GHSV = total volumetric flow rate dived by volume of catalyst bed) varied between 4014 and 4234 h−1. Total experimental run time averaged at 3 hours which generated 50 data points from the micro-GC, providing enough time for robust analysis of the mean syngas composition and the extent of carbon deposition. Experimental feed compositions can be found in Table 7. Each condition was tested in duplicate and the runs with the least variability used for analysis.
5 Simulation setup and NWaste2H2 process operation description
Aspen Plus® V.10 was used to simulate the novel H2 production process. An “ENRTL-RK” (Electrolyte Non-Random Two Liquid with Redlich-Kwong) base method were used throughout. Fig. 3 displays the full chemical processing plant as designed for this study. The ammonia recovery process is as that detailed in Grasham et al.15 with the exception that the flash separation unit was replaced with a distillation column. The air stripper was simulated using a RadFrac column with 30 trays, no condenser or reboiler, ‘standard’ convergence and using equilibrium (rather than rate-based) calculations. It is presumed that the digestate liquor has been treated with NaOH to increase the pH to a state in which all ammonia is contained in its free (NH3) form rather than as ionic ammonium. The recovery process begins with the introduction of digestate liquor (LIQ-1) which is preheated by exchange with the bottoms' exit from the stripper (STRIPPER). LIQ-2 then enters the top of the stripper (‘above stage 1’). Stripping air is compressed to 1.3 bar, pre-heated via two heat exchangers and a heater to 500 °C and fed to the bottom (‘on stage 30’) of the stripper. A 10% pressure drop was simulated through the stripping column.The ammonia is desorbed and released with air and a small amount of water vapour. This stream is cooled, allowing water to condense before entering an absorption column (ABSORB). The absorption column has also been simulated using a RadFrac column with 10 stages, no condenser or reboiler, using ‘standard’ convergence and equilibrium calculations. Here, a counter-flow stream of water (entering the top of the column above stage 1) meets the stripped material (entering the bottom of the column on stage 10) which allows the ‘recapturing’ of ammonia, leaving at the base of the absorber. This stream (EXIT-LIQ) is then fed into the distillation column (DIST) with 15 stages, a ‘partial-vapour’ condenser, kettle reboiler, using standard convergence and equilibrium calculations. The gaseous outlet from the distiller (CON-LIQA), containing a highly concentrated ammonia mixture is to be prepared for H2 production. The recovered ammonia stream is pressurised to 31 bar before mixing with a fraction of the distillation bottoms (BTMS2), the quantity of which is dictated by a pre-determined feed molar steam to carbon ratio (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) in the reformer, for which the carbon is from the biomethane co-feed. This combined stream, (CON-LIQC) is heated via a number of heat exchangers (HX6, HX5 and HX9) before entering the primary reformer/cracker (REFORMER) at 25 bar.
C) in the reformer, for which the carbon is from the biomethane co-feed. This combined stream, (CON-LIQC) is heated via a number of heat exchangers (HX6, HX5 and HX9) before entering the primary reformer/cracker (REFORMER) at 25 bar.
The reformer is simulated with an RGibbs reactor where the minimisation of Gibbs free energy is used to calculate the product yields and heat demand. Here, the pressurised recovered ammonia stream is met with pressurised and pre-heated bio-methane. At 1000 °C, the reformer generates a syngas formed primarily by a mixture of reactions (9–11). Reaction (9) describes ‘steam methane reforming’ (SMR) whereby methane is converted to H2 gas and carbon monoxide using high temperature steam. Reaction 10 displays ‘water gas shift’ (WGS) where steam is used to convert carbon monoxide to H2 gas and carbon dioxide. Reaction 11 exhibits the thermal decomposition/cracking of ammonia to H2 and nitrogen gas. S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C of 2, 3 and 4 were analysed in this work to determine the most advantageous conditions from both efficiency and economic points of view. S
C of 2, 3 and 4 were analysed in this work to determine the most advantageous conditions from both efficiency and economic points of view. S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C can be described as the molar ratio of H2O and CH4 entering the reformer.
C can be described as the molar ratio of H2O and CH4 entering the reformer.
The syngas produced from the primary reformer (SYN-1) expends heat via transfer with the reformer and furnace inlets in heat exchangers ‘HX1’ and ‘HX4’. It enters the high temperature shift HT-WGS (HT) and low temperature shift LT-WGS reactors (LT), also represented as RGibbs reactors, at 350 °C and 205 °C inlet temperatures respectively, for maximum CO conversion to CO2 and concurrent production of H2 from the water co-reactant via reaction (5). Each shift reactor operates adiabatically, so the exothermic nature of the WGS reaction creates a moderate temperature hike in each reactor. The syngas is then cooled and dried before a PSA unit generates a pure H2 product containing 90% of the H2 present in the PSA inlet.
| CH4 + H2O ⇆ 3H2 + CO | (9) |
| CO + H2O ⇆ H2 + CO2 | (10) |
| 2NH3 ⇆ 3H2 + N2 | (11) |
A furnace was used to generate heat for the process. It was simulated via an ‘RGibbs’ reactor (FURNACE) and is fuelled by a portion of the AD-biomethane product via the stream ‘CH4-FUEL’ together with any remaining H2, CH4 and CO present in the PSA's offgas (OFFGAS). The quantity of biomethane fed to the furnace is determined via a ‘Design Specification’ block which stipulates that the flow of methane should facilitate a post-reformer exhaust temperature of 1100 °C. The furnace then generates an excess of heat (heat duty) that equates to 110% of the reformer requirements. This fulfils the heat demand of the reformer and accounts for 10% thermal losses. A 10% pressure drop is experienced in each of the heat exchangers and reactors where the inlet pressure is slightly greater than atmospheric. Streams ‘EXHAUST3’, ‘UTILITY3’ and ‘UTILITY5’ shown in Fig. 3 provide the potential for heat export from the system. Their potential heat production have been calculated by cooling them to 23 °C and 1 bar via heater blocks in Aspen Plus® and given a 90% transfer efficiency factor.
A number of calculator blocks have been installed to the process model in order to fulfil associated process requirements, as detailed in eqn (12)–(15). Eqn (12) describes the requirement of an oxygen feed to the furnace to be 1.1 times that of the stoichiometric requirement. Eqn (13) is used to ensure that the ratio of nitrogen and oxygen in the air inlet remains at 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 on a molar basis. Eqn (14) ensures that the quantity of water entering as steam the primary reformer equates to the chosen steam to carbon ratio (S
21 on a molar basis. Eqn (14) ensures that the quantity of water entering as steam the primary reformer equates to the chosen steam to carbon ratio (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C). Eqn (15) was used to simulate the losses of heat (10%) from the furnace to the reformer. Amongst these equations:ṅx,y describes the molar flow of species ‘y’ in stream ‘x’ and HDFURNACE and HDREFORMER are the heat duties of the furnace and reformer respectively.
C). Eqn (15) was used to simulate the losses of heat (10%) from the furnace to the reformer. Amongst these equations:ṅx,y describes the molar flow of species ‘y’ in stream ‘x’ and HDFURNACE and HDREFORMER are the heat duties of the furnace and reformer respectively.
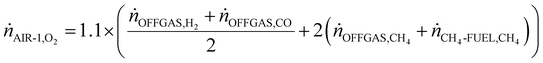 | (12) |
| ṅAIR−1,N2 = ṅAIR−1,O2 × 3.792 | (13) |
ṅBTMS2,H2O = (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) × ṅCH4−COMP,CH4 C) × ṅCH4−COMP,CH4 | (14) |
| HDFURNACE = −1.1 × HDREFORMER | (15) |
6 NWaste2H2 process modelling results
6.1 Ammonia recovery
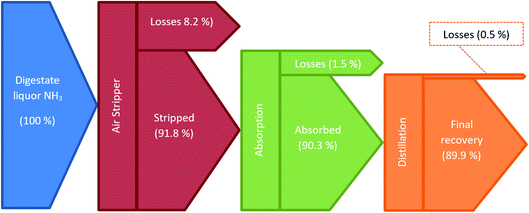
The ammonia recovery process showcased in this body of work closely resembles that exhibited in Grasham et al.15 However, as aforementioned, the flash separator has been replaced with a distillation column as sensitivity analysis proved a comparative energetic preference for distillation. Sensitivity analysis also demonstrated an energetic system preference for relatively low air flow rates at high temperatures for the air stripper (STRIPPER). This means an incoming air flow rate to the stripping in BOTAIR5 of 250 kmol h−1 (7213 kg h−1) at 550 °C and 1.1 bar. The liquor has ammonia and water flow rates of 2.95 kmol h−1 and 1528.6 kmol h−1 (50.3 and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 537.4 kg h−1) respectively, and is preheated to 64.1 °C via exchange with the stripper's bottom exit before entering the top of the stripper, thus facilitating an average column temperature of 64.3 °C. The result of these air stripping conditions is the recovery of 91.8% of the ammonia held in the initial digestate liquor.
537.4 kg h−1) respectively, and is preheated to 64.1 °C via exchange with the stripper's bottom exit before entering the top of the stripper, thus facilitating an average column temperature of 64.3 °C. The result of these air stripping conditions is the recovery of 91.8% of the ammonia held in the initial digestate liquor.
For the absorption step, a 145.5 kmol h−1 (3242.8 kg h−1) flow of water has been used, enabling the recovery of 98.4% of the ammonia entering the column. This translates to 90.3% of the digestate liquor's ammonia. This means the bottom exit of the absorber (EXIT-LIQ) contains 173.8 kmol h−1 of H2O and 2.67 kmol h−1 of NH3. The distillation column demonstrates only minimal losses, recovering 99.5% of the ammonia from the absorption outlet. Combined, the ammonia recovery system showcased an ability to recover 89.9% of the ammonia held in the digestate liquor, equating to 2.65 kmol h−1 (45.2 kg h−1). This is a considerable improvement on the 82% recovery potential demonstrated from the recovery process discussed in Grasham et al.15Fig. 4 illustrates the recovery and losses through each ammonia recovery step.
Any aqueous ammonia ‘losses’ via the stripper and distillation phases, as displayed in Fig. 4, will be diverted for conventional wastewater treatment with the rest of the digestate liquor. Gaseous ammonia is ‘lost’ during absorption in very small concentrations via a stream mainly consisting of air. This air can be used to supply the oxygen feed required in the WWTP's activated sludge process. These measures ensure there are no gaseous discharges of ammonia to the atmosphere and that any ammonia not undergoing reforming undergoes biological treatment.
6.2 H2 production
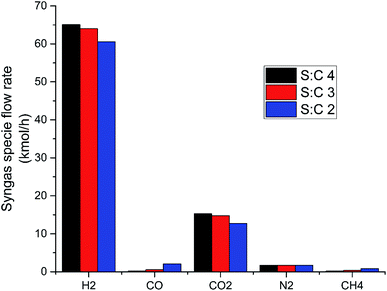
The flow of key syngas species from the primary reformer under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C conditions 2, 3 and 4 can be seen in Fig. 5. The most visible result present in Fig. 5 is the positive correlation between S
C conditions 2, 3 and 4 can be seen in Fig. 5. The most visible result present in Fig. 5 is the positive correlation between S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and H2 generation. This occurs due to Le Chatelier's principle whereby steam excess tips the SMR and WGS equilibria forward (reactions (9) and (10)), resulting in greater conversions of CO and CH4, and leaving lower quantities of these species in the syngas. The conversions of methane in the reformer were found to be 94.8, 97.9 and 98.9% for S
C and H2 generation. This occurs due to Le Chatelier's principle whereby steam excess tips the SMR and WGS equilibria forward (reactions (9) and (10)), resulting in greater conversions of CO and CH4, and leaving lower quantities of these species in the syngas. The conversions of methane in the reformer were found to be 94.8, 97.9 and 98.9% for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 respectively. Meanwhile, the conversions of CO were 16, 25.2 and 32.6% for S
C 2, 3 and 4 respectively. Meanwhile, the conversions of CO were 16, 25.2 and 32.6% for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 respectively. The conversion of ammonia in each S
C 2, 3 and 4 respectively. The conversion of ammonia in each S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenario showed very little variability, lying between 99.24–99.45%. This high conversion was expected due to the high temperature (1000 °C) being used in the reformer.
C scenario showed very little variability, lying between 99.24–99.45%. This high conversion was expected due to the high temperature (1000 °C) being used in the reformer.
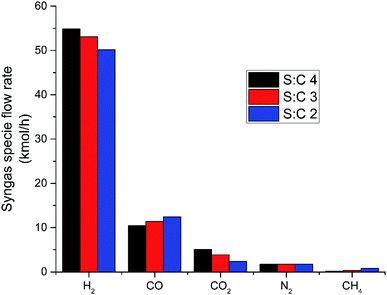 | ||
Fig. 5 Flow rate of key syngas species from the primary reformer for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios at 25 bar and 1000 °C. C 2, 3 and 4 scenarios at 25 bar and 1000 °C. | ||
The lower temperatures of the WGS reactors facilitate further conversion of CO via reaction 10. The HT-WGS reactor converts 51, 67 and 77% of the CO entering the reactor for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios 2, 3 and 4 respectively. The LT-WGS reactor sees conversions of 66, 86 and 91% of the CO entering the reactor downstream of the HT-WGS. Again, this positive correlation can be attributed to the excess steam tipping the equilibrium of the WGS reaction towards the products. The effect of this is the generation of an additional 10.3, 10.8 and 10.1 kmol h−1 of H2 for S
C scenarios 2, 3 and 4 respectively. The LT-WGS reactor sees conversions of 66, 86 and 91% of the CO entering the reactor downstream of the HT-WGS. Again, this positive correlation can be attributed to the excess steam tipping the equilibrium of the WGS reaction towards the products. The effect of this is the generation of an additional 10.3, 10.8 and 10.1 kmol h−1 of H2 for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios 2, 3 and 4 respectively. This indicates that although CO conversion percentages improved with higher S
C scenarios 2, 3 and 4 respectively. This indicates that although CO conversion percentages improved with higher S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio, the actual H2 increase was fairly constant due to the greater inlet of CO from the primary reformer under lower S
C ratio, the actual H2 increase was fairly constant due to the greater inlet of CO from the primary reformer under lower S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios.
C scenarios.
Fig. 6 highlights the impact of the WGS reactors on the composition of the syngas when compared to Fig. 5. CO has been significantly reduced, whilst CO2 and H2 have increased. The flows of H2 from the LT-WGS reactor in stream SYN-6 were 60.6, 64.0 and 65.1 kmol h−1 for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively. The outlet from the LT-WGS reactor is removed of most of its water content before entering the PSA which recovers 90% of the H2 contained in the syngas corresponding to 54.5 kmol h−1 (109.9 kg h−1), 57.6 kmol h−1 (116.1 kg h−1) and 58.6 kmol h−1 (118.1 kg h−1) for S
C 2, 3 and 4 scenarios respectively. The outlet from the LT-WGS reactor is removed of most of its water content before entering the PSA which recovers 90% of the H2 contained in the syngas corresponding to 54.5 kmol h−1 (109.9 kg h−1), 57.6 kmol h−1 (116.1 kg h−1) and 58.6 kmol h−1 (118.1 kg h−1) for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively.
C 2, 3 and 4 scenarios respectively.
6.3 Energetic implications
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios, the thermal demand for the reformer is more pronounced due to the higher volume of reactants requiring heating to maintain reactor temperature. This means that there is less heat to be recuperated in the upstream ammonia recovery. The heat demand of the AIRHT heater block, bringing air for stripping up to temperature, can be used as an indicator for how much heat is left after the H2 production. Table 2 details the heat demand for AIRHT under each S
C ratios, the thermal demand for the reformer is more pronounced due to the higher volume of reactants requiring heating to maintain reactor temperature. This means that there is less heat to be recuperated in the upstream ammonia recovery. The heat demand of the AIRHT heater block, bringing air for stripping up to temperature, can be used as an indicator for how much heat is left after the H2 production. Table 2 details the heat demand for AIRHT under each S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenario. It demonstrates that its energy requirement under the S
C scenario. It demonstrates that its energy requirement under the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 scenario is more than 2.5 times than under the S
C 4 scenario is more than 2.5 times than under the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 scenario.
C 2 scenario.
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 C 2 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 C 3 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 C 4 |
|
|---|---|---|---|
| Blocks | |||
| AIRHT | −296.1 | −615.0 | −781.0 |
| DIST | −365.5 | −365.5 | −365.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Streams | |||
| EXHAUST3 | 286.8 (125 °C) | 298.5 (146 °C) | 295.2 (148 °C) |
| UTILITY3 | 190.0 (57 °C) | 293.4 (75 °C) | 408.9 (95 °C) |
| UTILITY5 | 861.8 (63 °C) | 861.8 (63 °C) | 861.8 (63 °C) |
| Net total | 677.0 | 473.2 | 419.3 |
Table 2 also lists the heat demand of the distillation column's reboiler (365.5 kWth) and of the process streams that could be used as sources (EXHAST3, UTILITY3 AND UTILITY5). Although each of these streams were found to contain a considerable amount of thermal energy providing positive net thermal power figures, this heat is of low quality in each case, as displayed in Table 3. None of these streams are adequate to meet the high temperature requirements of the air stripper's inlet. This must therefore be met from external sources, which adds to the comparative attractiveness of using lower S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios used for reforming – from both energetic and economic vantage points. Although of low quality, the heat streams can be used on-site for purposes such as space-heating and anaerobic digestion.
C ratios used for reforming – from both energetic and economic vantage points. Although of low quality, the heat streams can be used on-site for purposes such as space-heating and anaerobic digestion.
| EXHAUST3 (°C) | UTILITY3 (°C) | UTILITY5 (°C) | |
|---|---|---|---|
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 C 2 |
65 | 125 | 63 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 C 3 |
88 | 146 | 63 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 C 4 |
120 | 148 | 63 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenario. A positive correlation between S
C scenario. A positive correlation between S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio and additional power requirements from process implementation can be seen. The key component of this audit is the electricity that would be needed for H2 product preparation during compression, storage and dispensing (CSD). The reason for this is that H2 would be pressurised to 350 bar for effective refuelling of high pressure tanks on-board the proposed fuel cell electric buses using the station. The CSD-derived power demand makes up over half of the additional power demand associated with the proposed process implementation.
C ratio and additional power requirements from process implementation can be seen. The key component of this audit is the electricity that would be needed for H2 product preparation during compression, storage and dispensing (CSD). The reason for this is that H2 would be pressurised to 350 bar for effective refuelling of high pressure tanks on-board the proposed fuel cell electric buses using the station. The CSD-derived power demand makes up over half of the additional power demand associated with the proposed process implementation.
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 C 2 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 C 3 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 C 4 |
|
|---|---|---|---|
| NWaste2H2 process | 3890 | 3907 | 3930 |
| Biogas clean up | 5239 | 5239 | 5239 |
| CSD | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 823 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 942 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 298 298 |
| N-diversion | −4120 | −4120 | −4120 |
| Net power use | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 832 832 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968 968 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 347 |
The impact on electricity use from the diversion of digestate liquor ammonia can also be seen in Table 4. By diverting and utilising this ammonia, additional energy requirements of process implementation could be reduced by over 20% within each S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio scenario. Nonetheless this is not enough to offset all of the energy needs that would come with process implementation. Accordingly, when adding the net power consumption figures displayed in Table 4, the referenced WWTP transforms from using an average 60 MWhelec per day to 84.8, 86 and 86.3 MW h pet day under S
C ratio scenario. Nonetheless this is not enough to offset all of the energy needs that would come with process implementation. Accordingly, when adding the net power consumption figures displayed in Table 4, the referenced WWTP transforms from using an average 60 MWhelec per day to 84.8, 86 and 86.3 MW h pet day under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively.
C 2, 3 and 4 scenarios respectively.
6.4 Greenhouse gas emission savings
There are two ways in which the operation of the process would release lifecycle GHG emissions, being the use of high-quality heat from natural gas and increased grid electricity consumption. Conversely, there are two methods that would lessen the lifecycle GHG footprint of the wastewater treatment facility; the diversion of ammonia present in digestate liquor from its conventional treatment process and abated emissions from diesel buses replaced with H2 fuel cell alternatives.
Table 5 indicates that impressive lifecycle GHG emission reductions would be possible with process implementation under each S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenario. It should be noted that these figures have been calculated with the assumption that there is no longer CHP technology in place at the WWTP. Abatement of air pollutant emissions via replacement of diesel-fuelled buses with H2 fuel cell alternatives would have the greatest impact on the overall environmental attractiveness of process implementation. These GHG savings are directly related to the production of H2 and is therefore more pronounced with higher S
C scenario. It should be noted that these figures have been calculated with the assumption that there is no longer CHP technology in place at the WWTP. Abatement of air pollutant emissions via replacement of diesel-fuelled buses with H2 fuel cell alternatives would have the greatest impact on the overall environmental attractiveness of process implementation. These GHG savings are directly related to the production of H2 and is therefore more pronounced with higher S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio utilisation in the process, as shown in Table 5.
C ratio utilisation in the process, as shown in Table 5.
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 C 2 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 C 3 |
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 C 4 |
|
|---|---|---|---|
| Net electricity | 2657 | 2779 | 2819 |
| High quality heat | 3335 | 4942 | 5779 |
| NH3 diversion | −3222 | −3222 | −3222 |
| Abated bus emissions | −37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964 964 |
−39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 693 |
−40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
| Total | −35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194 194 |
−35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195 195 |
−35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 414 414 |
The amount of high-quality heat required to perform effective air stripping and distillation was found to have a significant impact on reducing GHG emissions during process operation. The emissions resulting from the demand of high-quality heat under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 conditions outweigh the additional lifecycle emission reductions experienced via abated bus transport emissions when compared to the S
C 4 conditions outweigh the additional lifecycle emission reductions experienced via abated bus transport emissions when compared to the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario. This results in final GHG emission reductions of 35.20 tonnes CO2e per day under the S
C 3 scenario. This results in final GHG emission reductions of 35.20 tonnes CO2e per day under the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario which is 219 kg less than under S
C 3 scenario which is 219 kg less than under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 conditions. The S
C 4 conditions. The S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 scenario demonstrated the lowest lifecycle GHG emission reductions but just 1 kg CO2 per day less than under S
C 2 scenario demonstrated the lowest lifecycle GHG emission reductions but just 1 kg CO2 per day less than under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 conditions. For each person served by the facility, or population equivalent (PE), the total GHG savings found in Table 5 equate to 17.1, 17.1 and 17.2 kg CO2 per yr per PE for S
C 3 conditions. For each person served by the facility, or population equivalent (PE), the total GHG savings found in Table 5 equate to 17.1, 17.1 and 17.2 kg CO2 per yr per PE for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 conditions respectively.
C 2, 3 and 4 conditions respectively.
6.5 Economic sustainability assessment of NWaste2H2
The total initial capital investment requirements CAPEX, which includes equipment capital and installation costs for process implementation were found to be £12.53 million ($16.06 million), £13.01 million ($16.68 million) and £13.29 million ($17.04 million) for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively. The breakdown under the S
C 2, 3 and 4 scenarios respectively. The breakdown under the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario can be found in Table 6. The inter-scenario differences can mainly be attributed to the larger volume requirements of compression equipment under greater S
C 3 scenario can be found in Table 6. The inter-scenario differences can mainly be attributed to the larger volume requirements of compression equipment under greater S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C conditions. In each case roughly 41% of the initial investment was accredited to the fuel preparation compression, storage and dispensing (CSD) systems; costing £5.21 million, £5.35 million and £5.40 million for S
C conditions. In each case roughly 41% of the initial investment was accredited to the fuel preparation compression, storage and dispensing (CSD) systems; costing £5.21 million, £5.35 million and £5.40 million for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively. The greatest single unit investment cost from the discussed Aspen Plus® process was found to be CMPRS2 at £990
C 2, 3 and 4 scenarios respectively. The greatest single unit investment cost from the discussed Aspen Plus® process was found to be CMPRS2 at £990![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054.
054.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario
C 3 scenario
| Equipment | Installed cost (£) | Equipment | Installed cost (£) |
|---|---|---|---|
| C&S | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 789 789 |
PSA | 172![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 225 |
| CMPRS2 | 990![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 054 |
HX4 | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 526 |
| CMPRS3 | 988![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 416 |
COND1 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 942 |
| Gas clean up | 933![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688 688 |
HX5 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 304 304 |
| CMPRS1 | 577![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 902 |
HX10 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 758 |
| REFORMER | 552![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 690 |
COND2 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491 491 |
| AIRHT | 544![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076 |
HX7 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734 |
| HT | 458![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842 842 |
B10 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 486 |
| LT | 458![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842 842 |
HX8 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 374 |
| STRIPPER | 348![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 348 348 |
HX9 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 582 582 |
| DIST | 306![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072 072 |
HX6 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 694 |
| HX3 | 204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 126 |
HX1 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462 462 |
| HX2 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382 382 |
FURNACE | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
| ABSORP | 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 726 726 |
||
| Total | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011 011![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386 386 |
||
Fig. 7 displays the NPV of the discussed process over its projected 20 year lifetime for the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario. It demonstrates a discounted return on investment (i.e. when NPV equals 0) in 5.80 years which was the shortest of each S
C 3 scenario. It demonstrates a discounted return on investment (i.e. when NPV equals 0) in 5.80 years which was the shortest of each S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenario considered. The equivalent length of time under S
C scenario considered. The equivalent length of time under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 and 4 conditions were 6.13 and 5.96 respectively. This means that investors will see an average 10% annual return on their investment in under 6 years from its opening day for two of the three S
C 2 and 4 conditions were 6.13 and 5.96 respectively. This means that investors will see an average 10% annual return on their investment in under 6 years from its opening day for two of the three S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios. At a projected H2 value of £4.50 per kg, the associated (first year) income from H2 sales only would stand at £4.33 million, £4.58 million and £4.65 million for S
C scenarios. At a projected H2 value of £4.50 per kg, the associated (first year) income from H2 sales only would stand at £4.33 million, £4.58 million and £4.65 million for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios 2, 3 and 4 respectively. The sale of H2 as a transportation fuel is by far the greatest revenue stream, comprising roughly 84% of the income possible from process implementation. The rest of the income is made up of RTFO certificate sales (∼13%) and RHI (renewable heating incentive) payments (∼3%).
C scenarios 2, 3 and 4 respectively. The sale of H2 as a transportation fuel is by far the greatest revenue stream, comprising roughly 84% of the income possible from process implementation. The rest of the income is made up of RTFO certificate sales (∼13%) and RHI (renewable heating incentive) payments (∼3%).
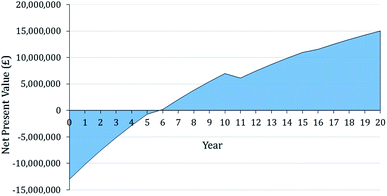 | ||
Fig. 7 Results of net present value over the 20 year lifetime of the novel H2 production plant under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 conditions. C 3 conditions. | ||
Fig. 8 illustrates the first-year operational costs (OPEX) after implementation of the system under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 conditions. It can be noted that the greatest expenditure is on grid electricity for the various power requirements at £1.16 million a year, equating to 46% of the total OPEX. Of spending on electricity, over two thirds can be attributed to the operation of the compression, storage and dispensing system. Whereas, the power requirements of the NWaste2H2 process (Fig. 3) contributes just 13% to the expenditure on grid electricity. The first year OPEX was found to be lowest in the S
C 3 conditions. It can be noted that the greatest expenditure is on grid electricity for the various power requirements at £1.16 million a year, equating to 46% of the total OPEX. Of spending on electricity, over two thirds can be attributed to the operation of the compression, storage and dispensing system. Whereas, the power requirements of the NWaste2H2 process (Fig. 3) contributes just 13% to the expenditure on grid electricity. The first year OPEX was found to be lowest in the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 scenario and highest in the S
C 2 scenario and highest in the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 scenario at £2.20 million, £2.34 million and £2.40 million for S
C 4 scenario at £2.20 million, £2.34 million and £2.40 million for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios 2, 3 and 4 respectively.
C scenarios 2, 3 and 4 respectively.
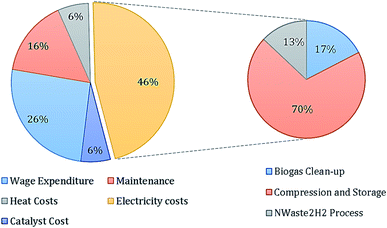 | ||
Fig. 8 Breakdown of first year OPEX for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario with a further breakdown of the expenditure on electricity. Total operational cost of £2.34 million. C 3 scenario with a further breakdown of the expenditure on electricity. Total operational cost of £2.34 million. | ||
After the 20 year lifetime of the plant, the implications of the yearly income and OPEX data is that the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario proved to be the most profitable of each scenario analysed. With final (20 years) NPV figures £14.08 million, £15.02 million and £15.00 million under S
C 3 scenario proved to be the most profitable of each scenario analysed. With final (20 years) NPV figures £14.08 million, £15.02 million and £15.00 million under S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 conditions respectively. This demonstrates that the low OPEX of the S
C 2, 3 and 4 conditions respectively. This demonstrates that the low OPEX of the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2 scenario would not outweigh the lower income potential, whilst the higher income potential of the S
C 2 scenario would not outweigh the lower income potential, whilst the higher income potential of the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 scenario would not outweigh its greater OPEX. For these reasons it would be suggested that the plant use an S
C 4 scenario would not outweigh its greater OPEX. For these reasons it would be suggested that the plant use an S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio of 3 in the primary reformer if the process was to be implemented at the reference WWTP.
C ratio of 3 in the primary reformer if the process was to be implemented at the reference WWTP.
Sensitivity analysis has been carried out on the economic study for the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario, to highlight factors that would considerably influence the profitability of implementation and operation. This sensitivity has been done for due diligence purposes. For example, variations in these economic parameters could occur if some costs have been heavily under/overestimated or severe weather occurs during construction or operation creating a spike in installation and operating costs. The results of the sensitivity analysis are displayed in Fig. 9. The tornado diagram demonstrates that a change in the price of catalyst would have minimal effect on the overall profitability with the implementation of the process at the reference WWTP, whilst a change in the market value of H2 would impact significantly the system's profitability.
C 3 scenario, to highlight factors that would considerably influence the profitability of implementation and operation. This sensitivity has been done for due diligence purposes. For example, variations in these economic parameters could occur if some costs have been heavily under/overestimated or severe weather occurs during construction or operation creating a spike in installation and operating costs. The results of the sensitivity analysis are displayed in Fig. 9. The tornado diagram demonstrates that a change in the price of catalyst would have minimal effect on the overall profitability with the implementation of the process at the reference WWTP, whilst a change in the market value of H2 would impact significantly the system's profitability.
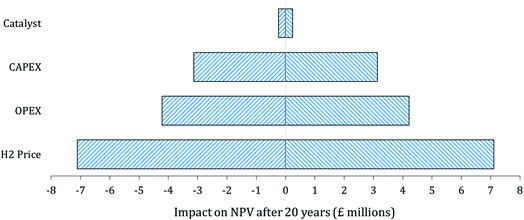 | ||
| Fig. 9 Difference in NPV (£ millions) after 20 years of operation with ±15% change in labelled factors. | ||
By decreasing the market value of H2 by 15%, the NPV after 20 years would be 47% lower. This is noteworthy considering the strong uncertainty in the future price of green H2. However, even with a 15% drop in its market value, a positive NPV (or discounted payback) could still be achieved in year 9 of operation and finish with an end-of-life NPV of +£7.9 million.
The H2 price detailed in this report is that of the ‘target price’ set by Reuter et al.40 for competitiveness with diesel rather than the current (2020) market value of H2 transport fuel in the UK which is at least £10 per kg. It is, therefore, speculated that the real market value of H2 should not fall below £4.50 per kg (at pump) for a considerable time. From a global perspective, it is expected the long-term market value will be determined by the cost of large-scale electrolysis and green electricity. If green electricity becomes so cheap that H2 price drops below £4.50 per kg (at pump) then the OPEX of the discussed process will also drop which would offset some of these proposed losses. Furthermore, if the market value of green electricity is significantly lower than it currently is, it may still be desirable to utilise biogas to produce higher-value products such as H2 rather than power generation.
Fig. 9 also illustrates that an increase or decrease in OPEX and CAPEX could have a significant impact on the financial attractiveness of process implementation. A 15% increase in OPEX and CAPEX (independently) would delay the achievement of a positive NPV by one and two years respectively; to the 7th and 8th years of operation. As a whole, the sensitivity analysis carried out on the economic study concluded that even with detrimental 15% alterations to various factors, implementation of the process could still be a commercially attractive move for the referenced wastewater treatment plant and thus those with similar characteristics.
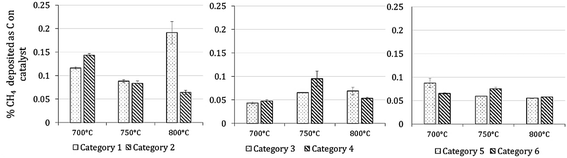
7 Results of H2 production from co-reforming methane and ammonia
Experimental analysis of combined steam methane reforming and ammonia decomposition was carried out to infer the tangible feasibility of the novel process at the core of the model. Table 7 describes the inlet compositions for the experiments and have been separated into categories 1–6. Categories 1–2 have (low) S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios between 2.18–2.32, categories 3–4 have (medium) S
C ratios between 2.18–2.32, categories 3–4 have (medium) S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios between 3.24–3.43 and categories 5–6 have (high) S
C ratios between 3.24–3.43 and categories 5–6 have (high) S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios between 4.31–4.47. The variation within categories was attributed to slight deviations in methane flows and unavoidable ammonia loss due to volatilisation during sample preparation. Pure SMR experiments are represented in odd numbered categories whilst combined SMR and ammonia decomposition experiments are found in the even numbered categories. The inlet flows in Table 7 were also used for equilibrium modelling for further comparative analysis.
C ratios between 4.31–4.47. The variation within categories was attributed to slight deviations in methane flows and unavoidable ammonia loss due to volatilisation during sample preparation. Pure SMR experiments are represented in odd numbered categories whilst combined SMR and ammonia decomposition experiments are found in the even numbered categories. The inlet flows in Table 7 were also used for equilibrium modelling for further comparative analysis.
| Run category | Temperature (°C) | CH4 (mol h−1) | H2O (mol h−1) | NH3 (mol h−1) | S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
|---|---|---|---|---|---|
| 1 | 700 | 0.049 | 0.106 | 2.18 | |
| 750 | 0.048 | 0.106 | 2.20 | ||
| 800 | 0.047 | 0.106 | 2.24 | ||
| 2 | 700 | 0.048 | 0.109 | 0.007 | 2.26 |
| 750 | 0.048 | 0.112 | 0.004 | 2.32 | |
| 800 | 0.048 | 0.109 | 0.007 | 2.29 | |
| 3 | 700 | 0.048 | 0.159 | 3.34 | |
| 750 | 0.049 | 0.159 | 3.26 | ||
| 800 | 0.049 | 0.159 | 3.24 | ||
| 4 | 700 | 0.048 | 0.163 | 0.007 | 3.39 |
| 750 | 0.048 | 0.165 | 0.005 | 3.43 | |
| 800 | 0.048 | 0.165 | 0.005 | 3.42 | |
| 5 | 700 | 0.049 | 0.212 | 4.33 | |
| 750 | 0.048 | 0.212 | 4.38 | ||
| 800 | 0.049 | 0.212 | 4.33 | ||
| 6 | 700 | 0.049 | 0.218 | 0.005 | 4.47 |
| 750 | 0.050 | 0.219 | 0.005 | 4.41 | |
| 800 | 0.050 | 0.215 | 0.008 | 4.31 |
Table 8 displays the results from the analysis of ammonia decomposition from the experiments combining ammonia decomposition and SMR. Ammonia conversions varied from 86 to 98%. Average NH3 conversions at each temperature analysed (700, 750 and 800 °C) evidenced a positive relationship between temperature and decomposition, as expected from the endothermic nature of reaction (11). As the equilibrium analysis showed >99% conversion of ammonia for each condition experimentally tested, this suggested experimental conditions getting closer to equilibrium of ammonia cracking with increasing reactor temperature. Future tests could be carried out using a lower GHSV in order to increase the residence time of gases over the catalyst. General electric suggest a common industry GHSV of 3000 h−1, providing rationale to test lower feed:catalyst ratios.45
| Run cat | Temp (°C) | NH3 conversion (%) |
|---|---|---|
| 2 | 700 | 92.5 |
| 2 | 750 | 91.0 |
| 2 | 800 | 98.0 |
| 4 | 700 | 90.2 |
| 4 | 750 | 86.0 |
| 4 | 800 | 91.9 |
| 6 | 700 | 84.9 |
| 6 | 750 | 91.6 |
| 6 | 800 | 95.9 |
| Mean | 700 | 89.2 |
| 750 | 89.5 | |
| 800 | 95.2 |
Fig. 10 to Fig. 13 illustrate the average molar flow rate of H2, CH4, CO and CO2 respectively in the syngas for each experiment alongside their equilibrium equivalent. Fig. 10 (I-III) shows the average H2 flow rate in the product gas under low (I), medium (II) and high (III) S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C conditions respectively. Experimental results from run categories 1 and 2 (low S
C conditions respectively. Experimental results from run categories 1 and 2 (low S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) at 700 °C exhibited the greatest difference in H2 production to equilibrium equivalents at 9.9% and 10.1% lower production rates respectively. The closest H2 production from the experiments got to the equilibrium equivalent was at 800 °C in run categories 3 and 4 (medium S
C) at 700 °C exhibited the greatest difference in H2 production to equilibrium equivalents at 9.9% and 10.1% lower production rates respectively. The closest H2 production from the experiments got to the equilibrium equivalent was at 800 °C in run categories 3 and 4 (medium S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) with average H2 flow rates just 5.2% less than their equilibrium equivalent for both categories.
C) with average H2 flow rates just 5.2% less than their equilibrium equivalent for both categories.
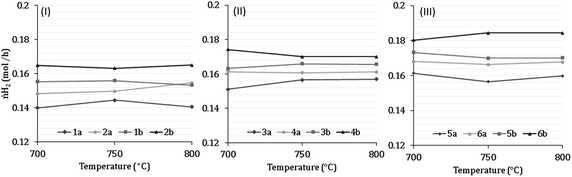 | ||
| Fig. 10 (I–III). Molar flow (mol h−1) of H2 in product gas from experimental (a) and equilibrium (b) runs. Inlet composition for run categories 1–6 can be found in Table 7. | ||
The slightly lower than equilibrium production of H2 can be attributed to the lower rates of SMR, WGS and ammonia decomposition reactions. Fig. 11 demonstrates how the flow of methane in the outlet of each experiment was slightly greater than predicted under equilibrium with the exception of run categories 5 and 6 (high S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios) at 800 °C where the flow of methane was under the micro GC's detectable limit. The experimental data displayed good correspondence with the equilibrium predictions in that methane conversions under the SMR reaction increased with both S
C ratios) at 800 °C where the flow of methane was under the micro GC's detectable limit. The experimental data displayed good correspondence with the equilibrium predictions in that methane conversions under the SMR reaction increased with both S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios and temperatures.
C ratios and temperatures.
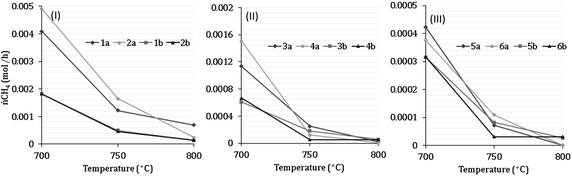 | ||
| Fig. 11 (I–III). Molar flow (mol h−1) of CH4 in product gas from experimental (a) and equilibrium (b) runs. Inlet composition for run categories 1–6 can be found in Table 7. | ||
The CO flow in the syngas produced during experiments and equilibrium studies can be observed in Fig. 12. With the exception of run category 2 at 700 °C, the flow of CO in the syngas was marginally greater in the experimental product compared to equilibrium, despite having lower rates of SMR. This was the case due to inhibited WGS (reaction 10), the effects of which are also evident when analysing the production of CO2 in Fig. 13. Experiment syngas CO2 flows were found to be 24% less than predicted by equilibrium for low S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C but just 4% less for medium S
C but just 4% less for medium S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C. The limited WGS reaction occurring during experiments, therefore had a significant impact on the final yield of H2.
C. The limited WGS reaction occurring during experiments, therefore had a significant impact on the final yield of H2.
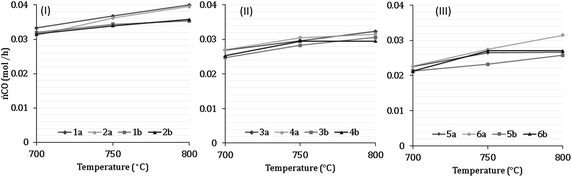 | ||
| Fig. 12 (I–III). Molar flow (mol h−1) of CO in product gas from experimental (a) and equilibrium (b) runs. Inlet composition for run categories 1–6 can be found in Table 7. | ||
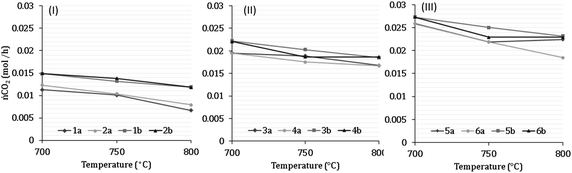 | ||
| Fig. 13 (I–III). Molar flow (mol h−1) of CO2 in product gas from experimental (a) and equilibrium (b) runs. Inlet composition for run categories 1–6 can be found in Table 7. | ||
It was hypothesised via equilibrium analysis that the presence of ammonia would have a slight negative impact on the conversion of methane via SMR due to the increased flow of H2 present in the syngas pushing the equilibrium of reaction 9 towards the reactant side. However, this was only found to be the case in just over half of the experiments. It was also hypothesised that carbon deposition would be less pronounced with the introduction of ammonia due to the occupation of acidic sites as discussed by Wang et al.46 However, no obvious correlation could be found, as illustrated in Fig. 14, where less than half of the runs with ammonia demonstrated lower rates of carbon deposition compared to the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C equivalents without ammonia.
C equivalents without ammonia.
Overall, the data displayed in Fig. 14 shows very little carbon deposition occurred in the experiments carried out. A slight negative correlation between carbon deposition and S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios was found, which is to be expected due to the role of steam in shifting the SMR reaction forwards and mediating the decomposition of methane to solid carbon and H2 (reaction 16) as the primary route of carbon formation at the investigated temperatures and pressure. For all experiments the carbon deposition was shown on average to be 0.075% of the methane molar flow. For the detailed discussed process, this would equate to 3.4 kg C per day. However, carbon formation is subject to kinetics, process condition and reactor design and these figures may not be representative to real working conditions, especially with the gap in pressure between the model and experiments.47 If the catalysts are deactivated via carbon formation, they may be regenerated via purging with steam or can be replaced altogether.
C ratios was found, which is to be expected due to the role of steam in shifting the SMR reaction forwards and mediating the decomposition of methane to solid carbon and H2 (reaction 16) as the primary route of carbon formation at the investigated temperatures and pressure. For all experiments the carbon deposition was shown on average to be 0.075% of the methane molar flow. For the detailed discussed process, this would equate to 3.4 kg C per day. However, carbon formation is subject to kinetics, process condition and reactor design and these figures may not be representative to real working conditions, especially with the gap in pressure between the model and experiments.47 If the catalysts are deactivated via carbon formation, they may be regenerated via purging with steam or can be replaced altogether.
| CH4 ⇆ 2H2 + C | (16) |
It has been established that combined ammonia decomposition and SMR can occur in a single packed-bed reactor using a commercial grade nickel catalyst and exhibited good correlation with equilibrium trends. The greater output of CO found experimentally would also help close the gap to equilibrium equivalents in a full H2 production system, where CO-shift reactors would generate the H2 proven to originate in the primary reactor under equilibrium. More importantly hardly any carbon deposition was found for operations with co-feeds of ammonia and methane at medium and high S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C. In this sense, the experimental results validate the model data with good confidence. Future work will refine operating conditions with analysis of alternative commercial catalysts, different WHSV/GHVSs to improve the reaction rates of eqn (9)–(11) and a gaseous inlet of ammonia to help control ammonia concentrations and S
C. In this sense, the experimental results validate the model data with good confidence. Future work will refine operating conditions with analysis of alternative commercial catalysts, different WHSV/GHVSs to improve the reaction rates of eqn (9)–(11) and a gaseous inlet of ammonia to help control ammonia concentrations and S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios.
C ratios.
Direct translation of the experimental results in the context of the modelling and economic analysis carried out is difficult due to the discussed disparity in exact thermodynamic conditions (model: 1000 °C, 25 bar, reactor: 800 °C, ∼1 bar). However, if H2 generation calculated for the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 case-study was 5.2% lower, as found with experimental results at atmospheric pressure and a temperature of 800 °C when compared to the equilibrium, a positive NPV would still be achieved during the 8th year of operation, just a year later than in the case-study, and a 20 year NPV 17.6% lower. Given the high discount factor used of 10%, it can be argued that these are still acceptable numbers for potential investors and wastewater treatment plant operators.
C 3 case-study was 5.2% lower, as found with experimental results at atmospheric pressure and a temperature of 800 °C when compared to the equilibrium, a positive NPV would still be achieved during the 8th year of operation, just a year later than in the case-study, and a 20 year NPV 17.6% lower. Given the high discount factor used of 10%, it can be argued that these are still acceptable numbers for potential investors and wastewater treatment plant operators.
8 Conclusions
To conclude, this work has presented the novel NWaste2H2 process in which ammonia is recovered from digestate liquor produced from anaerobic digestion at an operational wastewater treatment plant for use in a system that generates H2via a combination of ammonia decomposition and biomethane steam reforming. It was proposed that this H2 be used as vehicular fuel for fuel cell buses, replacing historically used diesel ones. The ammonia recovery system utilises air stripping, water absorption and distillation to produce a highly concentrated ammonia stream. The modelling of these process steps in Aspen Plus® exhibited a recovery potential of 89.9% which could then be used as a feedstock for H2 production.The primary H2 carrier used in the discussed process is bio-methane generated from anaerobic digestion of sewage sludge at the reference WWTP. Modelling of steam methane reforming was performed alongside ammonia decomposition in the system's primary reformer. Three scenarios with different feed molar S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C were simulated and analysed; S
C were simulated and analysed; S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C of 2, 3 and 4 under a reformer pressure 25 bar. As expected, the S
C of 2, 3 and 4 under a reformer pressure 25 bar. As expected, the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 scenario was capable of the highest methane conversion in the primary reformer at 98.9% compared to 94.8% and 97.9% for S
C 4 scenario was capable of the highest methane conversion in the primary reformer at 98.9% compared to 94.8% and 97.9% for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C scenarios 2 and 3 respectively. The high temperature conditions of the primary reformer (1000 °C) allowed for almost total decomposition of ammonia to H2 and N2. High and low temperature WGS reactors were utilised to manipulate the conversion of carbon monoxide to additional H2. After a PSA separation unit, this resulted in final H2 production figures of 109.9 kg h−1, 116.1 kg h−1 and 118.1 kg h−1 for S
C scenarios 2 and 3 respectively. The high temperature conditions of the primary reformer (1000 °C) allowed for almost total decomposition of ammonia to H2 and N2. High and low temperature WGS reactors were utilised to manipulate the conversion of carbon monoxide to additional H2. After a PSA separation unit, this resulted in final H2 production figures of 109.9 kg h−1, 116.1 kg h−1 and 118.1 kg h−1 for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively.
C 2, 3 and 4 scenarios respectively.
The price of generating additional H2 with higher S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios was an increase in heat and power requirements for the system. The heat streams generated were shown to be of insufficient quality to meet the high temperature demands of the air stripper and the distiller. The heat streams would be suitable for space and heat demand for anaerobic digestion at the facility but the quantity of heat available had an inverse relationship with S
C ratios was an increase in heat and power requirements for the system. The heat streams generated were shown to be of insufficient quality to meet the high temperature demands of the air stripper and the distiller. The heat streams would be suitable for space and heat demand for anaerobic digestion at the facility but the quantity of heat available had an inverse relationship with S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios as more heat was required for steam generation and then maintaining temperature in the primary reformer. Power usage had a positive relationship with the S
C ratios as more heat was required for steam generation and then maintaining temperature in the primary reformer. Power usage had a positive relationship with the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio with additional electricity required to compress both the reactants and H2 preparation for dispensing to H2-fueled buses at 350 bar.
C ratio with additional electricity required to compress both the reactants and H2 preparation for dispensing to H2-fueled buses at 350 bar.
The diversion of ammonia from conventional treatment was predicted to enable a significant drop in GHG emission at 3.2 tonnes CO2e per day, due to the reduced provision of oxygen required in biological nitrogen removal. Furthermore, GHG emission savings from the use of H2 as a bus transportation fuel were revealed to be 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964 kg CO2e, 39
964 kg CO2e, 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 kg CO2e and 40
693 kg CO2e and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 kg CO2e per day for S
790 kg CO2e per day for S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 2, 3 and 4 scenarios respectively, with the positive trend occurring due to augmented H2 generation at higher S
C 2, 3 and 4 scenarios respectively, with the positive trend occurring due to augmented H2 generation at higher S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios. This trend was partially offset by the increased emissions attributed to the use of external heat and power at higher S
C ratios. This trend was partially offset by the increased emissions attributed to the use of external heat and power at higher S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios. However, the S
C ratios. However, the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 4 scenario still demonstrated the highest potential reductions in lifecycle GHG emissions of the three S
C 4 scenario still demonstrated the highest potential reductions in lifecycle GHG emissions of the three S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C analysed, at 35
C analysed, at 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 414 kg CO2e per day, equivalent to 17.2 kg per year for each person served by the wastewater treatment plant.
414 kg CO2e per day, equivalent to 17.2 kg per year for each person served by the wastewater treatment plant.
Net present value analysis found that the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario had also the greatest economic promise with the lower OPEX. For the S
C 3 scenario had also the greatest economic promise with the lower OPEX. For the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 3 scenario, an initial total CAPEX of £13.01 million was required and showcased an ability to achieve a positive NPV during the 6th year of operation. Sensitivity analysis demonstrated that the profitability of the process would be highly influenced by the market value of H2, whereby a 15% drop in the proposed value of H2 (£4.50 per kg to £3.83 per kg) would lessen the 20 year NPV value by 47%.
C 3 scenario, an initial total CAPEX of £13.01 million was required and showcased an ability to achieve a positive NPV during the 6th year of operation. Sensitivity analysis demonstrated that the profitability of the process would be highly influenced by the market value of H2, whereby a 15% drop in the proposed value of H2 (£4.50 per kg to £3.83 per kg) would lessen the 20 year NPV value by 47%.
A key finding was the heavy influence that compression, storage and dispensing has on the CAPEX and OPEX of the system. In future work, alternative end uses of H2 will be analysed, such as injection into the UK's National Grid, which has been proposed as a possible option to decrease GHG emissions from the UK heat sector. The feasibility of process implementation at other anaerobic digestion facilities and WWTPs of different scales will also be assessed to interpret the potential role of the discussed process in the UK's energy landscape.
Experimental analysis validated the capability of combining the steam reforming of methane and the decomposition of ammonia in a single packed-bed reactor using a commercial grade 18 wt% NiO/α-Al2O3 catalyst. H2 yields reached between 90–95% of the equilibrium equivalent and ammonia decomposition exhibited higher sensitivity to temperature and steam to carbon ratio with conversions between 86% and 98%. Future work will concentrate on the refining of experimental conditions including analysis of alternative catalysts, and WHSVs, whilst a gaseous flow of ammonia will be used over an aqueous solution to bring more control to the experiments.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the UK's Engineering and Physical Sciences Research Council (EPSRC) for their grant award NWaste2H2 EP/R00076X/1. Further recognition to the EPSRC Centre for Doctoral Training in Bioenergy (EP/L014912/1) for the financial support of Mr Oliver Grasham with his PhD Scholarship. The authors' gratitude also extends to Yorkshire Water for allowing access to their facilities for the collection of samples and to Twigg Scientific Ltd. for their supply of catalyst.References
- I. A. Gondal, S. A. Masood and R. Khan, Int. J. Hydrogen Energy, 2018, 43, 6011–6039 CrossRef CAS.
- R. Karolyte, Hydrogen with CCS for decarbonised heat in the Scottish context, 2017 Search PubMed.
- A. Demirbas, Energy Sources, Part B, 2017, 12, 172–181 CrossRef CAS.
- N. P. Brandon and Z. Kurban, Philos. Trans. R. Soc., A, 2017, 375, 20160400 CrossRef.
- E. S. Hanley, J. P. Deane and B. P. Ó. Gallachóir, Renewable Sustainable Energy Rev., 2018, 82, 3027–3045 CrossRef.
- G. Maggio, A. Nicita and G. Squadrito, Int. J. Hydrogen Energy, 2019, 44, 11371–11384 CrossRef CAS.
- A. M. Abdalla, S. Hossain, O. B. Nisfindy, A. T. Azad, M. Dawood and A. K. Azad, Energy Convers. Manage., 2018, 165, 602–627 CrossRef CAS.
- M. Reinders, P. Beckhaus, F. Illing, U. Misz, H. Riße, M. Schröder, P. Schulte and B. Teichgräber, Int. J. Hydrogen Energy, 2015, 40, 8601–8606 CrossRef CAS.
- H. J. Alves, C. Bley Junior, R. R. Niklevicz, E. P. Frigo, M. S. Frigo and C. H. Coimbra-Araújo, Int. J. Hydrogen Energy, 2013, 38, 5215–5225 CrossRef CAS.
- S. Hwangbo, K. J. Nam, J. Han, I. B. Lee and C. K. Yoo, Energy Trends, 2018, 140, 386–397 CAS.
- Department for Business Energy & Industrial Strategy, National Statistics, DOI:10.4324/9780203732861-2.
- C. Vaneeckhaute, V. Lebuf, E. Michels, E. Belia, P. A. Vanrolleghem, F. M. G. Tack and E. Meers, Waste Biomass Valorization, 2016, 1–20 Search PubMed.
- M. Errico, L. Fjerbaek Sotoft, A. Kjærhuus Nielsen and B. Norddahl, Clean Technol. Environ. Policy, 2017, 20, 1479–1489 CrossRef.
- Agriculture and Horticulture Development Board (AHDB), GB Fertiliser Price Market Update Monthly average prices for June 2020 GB Fertiliser Price Market Update, 2020 Search PubMed.
- O. Grasham, V. Dupont, M. A. Camargo-Valero, P. García-Gutiérrez and T. Cockerill, Appl. Energy, 2019, 240, 698–708 CrossRef CAS.
- CEC – Council of the European Communities, Off. J. Eur. Communities: Legis., 1991, 135, 40–52 Search PubMed.
- CEC – Council of the European Communities, Off. J. Eur. Communities: Legis., 2000, 327, 1–72 Search PubMed.
- J. M. Garrido, M. Fdz-Polanco and F. Fdz-Polanco, Water Sci. Technol., 2013, 67, 2294 CrossRef CAS.
- M. J. Kampschreur, H. Temmink, R. Kleerebezem, M. S. M. Jetten and M. C. M. van Loosdrecht, Water Res., 2009, 43, 4093–4103 CrossRef CAS.
- J. Andrews, B. Chambers, A. Davey, S. Galetti, J. Hobson, D. Hunt, R. Thorman and I. Walkerm, Carbon Accounting in the Water Industry; non-CO2 Emissions, UKWIR, London, 2009 Search PubMed.
- V. Parravicini, K. Svardal and J. Krampe, Energy Procedia, 2016, 97, 246–253 CrossRef CAS.
- R. Berger, Fuel Cell Electric Buses – Potential for Sustainable Public Transport in Europe FCH JU – Commercialisation Strategy for Fuel Cell Electric Buses in Europe, 2015 Search PubMed.
- B. Roland, Fuel Cells and Hydrogen for Green Energy in European Cities and Regions, 2018 Search PubMed.
- The Hydrogen Council, Path to hydrogen competitiveness a cost perspective, 2020 Search PubMed.
- IEA, Hydrogen, Paris, 2020 Search PubMed.
- V. Willmann, LowCVP Low Emission Bus Workshop, Glasgow, 08/03/2018 Search PubMed.
- C. Hatch, A. Center, A. S. Feitelberg, E. M. Fisher and P. F. Mutolo, Int. J. Hydrogen Energy, 2013, 38, 16002–16010 CrossRef CAS.
- Department of Business Energy & Industrial Strategy, 2019 Government greenhouse gas conversion factors for company reporting: Methodology paper, London, 2019 Search PubMed.
- Aspen Plus, Aspen Technology, 2017 Search PubMed.
- AWWA, in Standard methods for the examination of water and wastewater, 1999 Search PubMed.
- G. Mininni, G. Laera, G. Bertanza, M. Canato and A. Sbrilli, Environ. Sci. Pollut. Res. Int., 2015, 22, 7203–7215 CrossRef CAS.
- N. Hill, E. Bonifazi, R. Bramwell, B. Karagianni and B. Harris, Government GHG Conversion Factors For Company Reporting: Methodology paper for emission factors: final report, 2018, p. 141 Search PubMed.
- Department for Business Energy & Industrial Strategy, 2019 Government Greenhouse Gas Conversion Factors for Company Reporting: Methodology Paper for Emission Factors Final Report, Stationary Office, London, 2019 Search PubMed.
- Department of Business Energy & Industrial Strategy, Updated Energy and Emissions Projections 2017, London, 2018 Search PubMed.
- Parliamentary Office of Science and Technology, Carbon Footprint of Heat Generation, 2016, vol. 523 Search PubMed.
- Aspen Technology, Aspen Plus Economic Analyzer, 2017 Search PubMed.
- M. Penev, G. Saur, C. Hunter and J. Zuboy, H2A Hydrogen Production Model: Version 3. 2018 User Guide (DRAFT), 2018 Search PubMed.
- M. S. Peters, K. D. Timmerhaus and R. E. West, in Plant design and economics for chemical engineers, McGraw-Hill, New York, 5th edn, 2004, pp. 642–754 Search PubMed.
- L. Kaiwen, Y. Bin and Z. Tao, Energy Sources, Part B, 2018, 13, 109–115 CrossRef.
- B. Reuter, M. Faltenbacher, O. Schuller, N. Whitehouse and S. Whitehouse, New Bus ReFuelling for European Hydrogen Bus Depots: High-Level Techno-Economic Project Summary Report, 2017 Search PubMed.
- Department of Business Energy & Industrial Strategy, Gas and electricity prices in the non-domestic sector, 2018 Search PubMed.
- W. A. Alkhayat and A. M. Gerrard, in Transactions of the American Association of Cost Engineers (Trans Am Assoc Cost Eng Annu Meet), Montreal, Canada, 1984, pp. 1.2.1–1.2.4 Search PubMed.
- R. Turton, R. C. Bailie, W. B. Whiting and J. A. Shaeiwitz, in Analysis, Synthesis, and Design of Chemical Processes, ed. R. Turton, R. C. Bailie, W. B. Whiting and J. A. Shaeiwitz, Prentice Hall, Upper Saddle River, N.J., 3rd edn, 2008 Search PubMed.
- The United Kingdom Department of Transport, Renewable Transport Fuel Obligation Guidance Part One Process Guidance Moving Britain Ahead, London, 2018 Search PubMed.
- W. Wei and K. Liu, presented at the 2007 Bio-Derived Liquids to Hydrogen Distributed Reforming Working Group held November 6, 2007 in Laurel, Maryland, GE Global Research, DOI:10.1017/CBO9781107415324.004.
- W. Wang, R. Ran, C. Su, Y. Guo, D. Farrusseng and Z. Shao, J. Power Sources, 2013, 240, 232–240 CrossRef CAS.
- J. N. Armor, Appl. Catal., A, 1999, 176, 159–176 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |