Evaluation of different Ni–semiconductor composites as electrodes for enhanced hydrogen evolution reaction†
Abstract
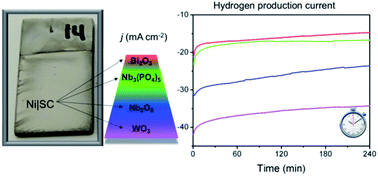
The use of earth-abundant materials for designing efficient and stable electrocatalysts is of paramount importance to facilitate large-scale production of hydrogen. In this work we developed a new series of electrodes based on Ni–semiconductor composites (Ni|SC) that are easy to synthesize (binder-free, economic and readily scalable method of synthesis), highly stable and active towards electrochemical hydrogen production under alkaline conditions. We showed the direct electrodeposition of composites (Ni|SC) from nickel-Watts plating baths modified by the addition of Nb2O5, Nb3(PO4)5, Bi2O3 and WO3 semiconductor particles. The electrodes were characterized by different techniques (electron and confocal microscopy, X-ray spectroscopy, X-ray diffraction, Raman spectroscopy, among others) before and after their electrochemical evaluation as catalysts for hydrogen evolution from water. In order to gain insights into their structure–activity relationship, the materials were also characterized by means of electrochemical analyses, i.e., cyclic voltammetry, chronoamperometry and electrochemical impedance spectroscopy. All catalysts have onset potential values around −1.1 V vs. SCE and similar Tafel slopes (ca. 120 mV dec−1) corresponding to the Volmer reaction as the rate determining step of the reaction. These catalysts show an increase of up to 115% (for Ni|WO3) of hydrogen production current compared to conventional Ni catalysts, in most cases preserving great chemical and structural stability after short ageing under alkaline conditions. The composite catalysts were synthesized on low-cost nickel-plated stainless-steel supports, which make them excellent alternatives for replacing massive nickel electrodes in conventional alkaline electrolyzers.


 Please wait while we load your content...
Please wait while we load your content...