Engineering nanostructured spinel ferrites by co-substitution for total water electrolysis by preferential exposure of metal cations on the surface†
Abstract
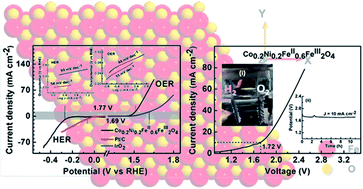
Single phasic magnetite samples co-substituted with cobalt and nickel, with the formula CoxNi(0.4−x)FeII0.6FeIII2O4 (x = 0, 0.1, 0.2, 0.3, and 0.4) were synthesized in the nanoregime via a co-precipitation technique. Being an inverse spinel, magnetite will preferentially expose the octahedral sites and make metal cations available on the surface, which will play a conducive role in both hydrogen evolution (HER) and oxygen evolution (OER) reactions. We demonstrate that the partial substitution of Fe2+ (either by Ni2+ or Co2+ ions) on the octahedral sites of the inverse spinel structure of CoxNi(0.4−x)FeII0.6FeIII2O4 significantly enhance the bifunctional electrocatalytic activity of the magnetite samples in an alkaline medium. The spinel ferrite with the formula Co0.2Ni0.2FeII0.6FeIII2O4 exhibits outstanding bifunctional electrocatalytic activity in 1 M KOH with the lowest onset overpotential (ƞOER = 190 and ƞHER = 200 mV), small overpotential at η10 (OER = 270 mV and HER = 275 mV), excellent kinetics (Tafel slopes, bOER = 44 mV dec−1 and bHER = 99 mV dec−1), and high durability (>10 h). Furthermore, Co0.2Ni0.2FeII0.6FeIII2O4 can serve as both cathode and anode for the overall water-splitting reaction, and delivered a current density of 10 mA cm−2 at a very low cell voltage of 1.72 V with excellent stability (>10 h at 10 mA cm−2). Thus, this work provides a lucid approach to engineer a highly efficient non-noble transition metal-based electrocatalyst for renewable energy applications via simple micro-structural and surface engineering.


 Please wait while we load your content...
Please wait while we load your content...