Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Industrial feasibility of anodic hydrogen peroxide production through photoelectrochemical water splitting: a techno-economic analysis†
Kasper
Wenderich
 *a,
Wouter
Kwak
a,
Alexa
Grimm
b,
Gert Jan
Kramer
b,
Guido
Mul
*a,
Wouter
Kwak
a,
Alexa
Grimm
b,
Gert Jan
Kramer
b,
Guido
Mul
 a and
Bastian
Mei
a and
Bastian
Mei
 *a
*a
aMESA+ Institute, Photocatalytic Synthesis Group, University of Twente, P. O. Box 217, 7500 AE Enschede, The Netherlands. E-mail: k.wenderich@utwente.nl; b.t.mei@utwente.nl; Tel: +31-53-4891985
bUtrecht University, Princetonlaan 8a, 3584 CB Utrecht, The Netherlands
First published on 17th April 2020
Abstract
Photoelectrochemical (PEC) water splitting is a promising approach to drive green, carbon-free production of hydrogen (H2). In ‘classic’ water splitting, oxygen (O2) is formed at the anode as a by-product. It has been suggested that substitution of anodic O2 production with hydrogen peroxide (H2O2) could increase the financial attractiveness of PEC water splitting. Here, we present a techno-economic analysis of a photoelectrochemical H2/H2O2 process. Specifically, we model photoelectrochemical farms with industrially relevant production capacities. Two scenarios are considered: (i) a theoretical scenario with an optimal solar-to-hydrogen (STH) efficiency of 27.55% and (ii) a literature-based state-of-the-art scenario with an STH efficiency of 10.1%. When applying an averaged market value of $0.85 kg−1 for H2O2, the analysis reveals a negative levelized cost of hydrogen (LCH) for scenario (i), i.e. $6.45 kg−1, and for scenario (ii) an LCH of $6.19 kg−1. Our results imply that these values are superior to the LCH of ‘classic’ PEC water splitting (ca. $10 kg−1), while the negative value for scenario (i) even outcompetes the LCH of steam methane reforming ($1.4 kg−1). We predict that significant reduction in the LCH can be realized within the PEC community when future research is aimed at enhancing the stability of the photoanode and optimizing the STH efficiency for anodic H2O2 formation. This manuscript clearly demonstrates the financial benefits of value-added product formation, such as hydrogen peroxide, over O2 formation. In a broader context, our analysis verifies that further research on valuable commodity chemicals at the anode in water splitting and CO2 reduction should be stimulated in the future to facilitate implementation of emerging, cost-intensive technologies.
Introduction
In the last few decades, interest in light-driven water splitting has been increasing rapidly.1–3 Electrochemical water splitting is foreseen to enable an environment-friendly way of harvesting and storing energy from renewable sources, such as solar energy, in the form of “green” hydrogen. In typical scenarios, hydrogen is produced in a (photo)electrochemical cell at the cathode by proton or water reduction in respectively acidic or alkaline media. Eqn (1) demonstrates the half-reaction for proton reduction:| 2H+ + 2e− → H2 E0(H+/H2) = 0 V vs. RHE | (1) |
Meanwhile, at the anode oxidation of water (acidic media) or hydroxide (alkaline media) takes place. The half-reaction for water oxidation is given by:
| 2H2O → O2 + 4H+ + 4e− E0(O2/H2O) = 1.23 V vs. RHE | (2) |
The overall reaction becomes:
| 2H2O → 2H2 + O2 E0cell = −1.23 V | (3) |
In the context of light-driven water splitting, two approaches are generally considered. In PV-E light harvesting, photovoltaic (PV) cells are coupled with water electrolysis. Generally, PV-E is considered as an appealing solution, as it allows for individual optimization of light harvesting for electricity generation and for fuel/chemical production by electrolysis. As an alternative, direct utilization of solar energy in a photoelectrochemical (PEC) cell is frequently discussed.4 With regard to techno-economic analyses, there is no consensus in literature whether PV-E or PEC provides the lowest average price of H2. For example, Shaner et al. estimate the average levelized cost of hydrogen (LCH) to be $12.1 kg−1 and $11.4 kg−1 for base-case PV-E and PEC systems respectively (with plant efficiencies of ca. 10%),4 whereas more recently Grimm et al. predicted these prices to be $6.22 kg−1 and $8.43 kg−1 (with solar-to-hydrogen efficiencies of 10.9 and 10% respectively).5 Often, for PEC water splitting, the average price of H2 is around $10 kg−1 for systems providing solar-to-hydrogen (STH) efficiencies around 10%.4,6,7 So far, hydrogen produced by PEC water splitting cannot compete yet with hydrogen produced by steam methane reforming (SMR), which has a current market value of $1.4 kg−1 H2 produced.4,8 Development of stable, non-toxic and efficient semiconductor materials to facilitate fabrication of systems with higher STH efficiencies seems to be a straight-forward approach to invoke more industrial interest in environmental-friendly photoelectrochemical water splitting.9,10
In a ‘classic’ (photo)electrochemical water splitting process oxygen is produced as anodic by-product (eqn (2)). With a market value of only $35 ton−1, oxygen is of low commercial interest and barely contributes to reduce the levelized cost of hydrogen (LCH).8 Therefore, another strategy to render PEC hydrogen production more attractive is to develop processes in which valuable products are formed at the anode.
A very alluring chemical to produce is hydrogen peroxide (H2O2). H2O2 is an important, environmental-friendly oxidant used for e.g. pulp and textile bleaching, disinfection, detergents, wastewater or exhaust air treatment. It is also used in chemical synthesis, semiconductor cleaning and it can be utilized in a fuel cell.11–15 As of 2015, 5.5 million tonnes of H2O2 are produced annually,13 mostly through the two-step anthraquinone process.11–18 In this process, anthraquinone is hydrogenated using e.g. nickel or supported palladium catalysts. The hydroquinones formed are subsequently oxidized with air, yielding H2O2 and regenerated anthraquinone. Afterwards, water is used for H2O2 extraction and distillation is applied to concentrate the H2O2. Despite its high usage in industry, the anthraquinone process suffers from some major drawbacks, including (but not limited to) the need for centralized production and the requirement of harmful organic solvents. A solution to these drawbacks could be the (photo)electrochemical production of hydrogen peroxide through selective two-electron oxidation of water (eqn (4)):8
| 2H2O → H2O2 + 2H+ + 2e− E0(H2O2/H2O) = +1.78 V vs. RHE | (4) |
In such case, the overall water splitting reaction becomes:
| 2H2O → H2O2 + H2 E0cell = −1.78 V | (5) |
This allows for simultaneous stoichiometric production of H2. Furthermore, such a process would allow for on-site production of H2O2 rather than centralized production without the need for any harmful solvents. In such a way, the need for extended transportation of a perilous substance sensitive to degradation is eliminated.11,12,14–19 With a current market value of $500–1200 ton−1 H2O2,8,20 hydrogen evolution with the co-production of H2O2 through selective water oxidation can significantly contribute to LCH reduction. This approach can therefore be a significant economic driver for H2-PEC development.
In 1853, Meidinger already demonstrated that hydrogen peroxide could be produced electrochemically by the electrolysis of sulfuric acid.11,16,21,22 Here, peroxodisulfuric acid is formed as an intermediate, which is hydrolyzed by water to eventually yield sulfuric acid and hydrogen peroxide.23 In recent years, the interest in electrochemical H2O2 formation has been rekindled, where selective water oxidation has been reported both by theory and experiments.18,24–41 For example, MnOx was demonstrated to work as an anode for selective water oxidation to H2O2.31 Although further material development is still required, in more recent studies metal oxides such as WO3 and BiVO4 seem to be excellent electrode materials, providing high selectivities for selective water oxidation to H2O2 at reasonable overpotentials (roughly 200 and 350 mV respectively).29,33 The favorable selectivity is governed by the binding energies of OH* and O* intermediates. Specifically, when ΔGO ≳ 3.5 eV and ΔGOH ≲ 2.4 eV, theory predicts that H2O2 should be the main water oxidation product. Pioneering work by Sayama et al. and Fuku et al.24–27,30 confirmed that BiVO4 is indeed a well-suited material for the production of H2O2. Faradaic efficiencies (FE) of up to 80% at an applied potential of 1.5 V under simulated solar light were obtained.27 Similarly, Shi et al. observed faradaic efficiencies ranging from ca. 63% up to 98% at additional potentials of 1.5 and 1.9 V vs. RHE respectively under (simulated) solar illumination.29 Moreover, Gd:BiVO4,38 surface phosphate-treated Mo:BiVO4,39 ZnO,40 CaSnO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 germanium porphyrins42 and aluminum porphyrins43 have been shown recently to be interesting materials for selective water oxidation to H2O2 as well. Lastly the inclusion of a protective layer such as mesoporous and amorphous Al2O3 could prevent anodic H2O2 degradation to O2, thus enhancing selectivity.27 For further reading, we refer the reader to one of the excellent reviews published on this topic.11,44,45
41 germanium porphyrins42 and aluminum porphyrins43 have been shown recently to be interesting materials for selective water oxidation to H2O2 as well. Lastly the inclusion of a protective layer such as mesoporous and amorphous Al2O3 could prevent anodic H2O2 degradation to O2, thus enhancing selectivity.27 For further reading, we refer the reader to one of the excellent reviews published on this topic.11,44,45
Based on the presented examples it can be concluded that significant advances have been made in the development of (photo)electrochemical H2O2-production by selective water oxidation. Integration in PEC devices is clearly of interest. Still, the question arises whether photoelectrochemical water splitting with selective hydrogen peroxide formation is financially attractive. Along these lines, Palmer et al. reported that the anodic production of commodity chemicals, such as iodine and bromine, through solar approaches can financially be more rewarding than anodic oxygen evolution via PEC water splitting.46 Here, we perform an in-depth techno-economic analysis of a H2 and H2O2 generating PEC system that, to the best of our knowledge, is still missing. We use an optimized system, i.e. we consider that the required semiconductors are readily available and reactions occur with 100% faradaic efficiencies to allow for maximum solar-to-hydrogen (STH) efficiencies resembling theoretical calculations.8 For such systems, we calculate the levelized cost of hydrogen (LCH) for coupled H2/H2O2 configurations as a function of H2O2 price. This system will be referred to as scenario (i). In addition, despite the novelty and therefore uncertainty, we present in scenario (ii) the techno-economics of a H2/H2O2 PEC cell using recent literature data reported by Shi et al.,29 using BiVO4 as a photoanode. To provide the required cell voltage, additional photovoltaic assistance is used. A strong dependence of LCH on the H2O2 price is observed. Furthermore, we predict through a sensitivity analysis that the LCH can significantly be reduced when the photoanode stability and the STH efficiency are enhanced. Importantly, for both models, we find that with reasonable H2O2 prices, the levelized cost of hydrogen is significantly lower in a H2/H2O2 PEC configuration than in a H2/O2 PEC configuration. From a financial point of view concomitant H2O2 production is beneficial, and our results highlight that research to facilitate anodic H2O2 production should thus be stimulated.
Methodology
Overall system design
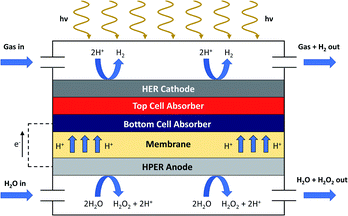
In scenario (i), we adapt the PEC module geometry of a fixed panel array reactor as described by Pinaud et al.6 and James et al.7 The fixed panel arrays are fully integrated devices and consist of two electrodes with multiple photoactive layers stacked between them. Specifically, we assume that the photoactive layer consists of two photoabsorbers with matching band gaps to allow for optimized STH efficiencies. One of the electrodes is used as a cathode for the hydrogen evolution reaction (HER), whereas the other electrode serves as an anode for the hydrogen peroxide evolution reaction (HPER). As a high pH is detrimental for the stability of H2O2, we use acidic conditions in our model.11,16,19 A membrane is introduced between the bottom cell absorber and the anode to allow the transition of protons and separation of the cathode and anode compartments. The cathode and the anode are exposed to a continuous flow of respectively wet gas and water. A schematic depicting the fixed panel array used in our research is shown in Fig. 1. In previous work,8 a web-based model (WBM) developed by Seger et al.47 was used to demonstrate that STH efficiencies of 27.55% can be reached when the top cell and bottom cell absorber have bandgaps of 1.9 eV and 1.2 eV respectively (in 1.0 M KOH, at 150 mV overpotential and with faradaic efficiencies of 100% for H2 and H2O2 generation). Here, using the same WBM and a similar approach (with 100% faradaic efficiencies for H2 and H2O2 generation), we derive that the maximum STH efficiency in 1.0 M H2SO4 and at 150 mV overpotential is also 27.55%, with the top and bottom cell absorbers being 1.9 and 1.2 eV as well (for additional information see ESI†).A schematic representation of the integration of the PEC module into an industrial process is depicted in Fig. 2. The anode is constantly fed with fresh water during operation. After reaction, O2 and a H2O/H2O2 mixture are obtained from the water and the two products are separated. Here, the option for water evaporation to concentrate the H2O2 is considered as well. This is realized by distillation or rectification at moderate temperatures and low pressures.16,48 It should be noted that for some applications of on-site production of H2O2, further concentrating of hydrogen peroxide is not needed. Here, the water is recycled afterwards. Also the electrolyte is recycled; for example, when sulfuric acid is used, steam can be used to remove water and hydrogen peroxide from the solution, similar to the Degussa–Weissenstein process.16 The resulting vapor is guided to a fractionating column, where water and hydrogen peroxide are separated. Hydrogen peroxide solution will form the output of the H2/H2O2 PEC plant, whereas the separated water will be reused. Alternatively, calcium hydroxide can be used for the precipitation of poorly soluble calcium sulfate.49 The precipitate is then desulfurized by thermal dissociation to form sulfur dioxide, which can be converted to sulfuric acid.50 At the cathode, a wet gas purge is used to harvest the generated hydrogen. Consequently, the hydrogen is separated and pressurized. The wet gas is recycled as well. Separation of chemicals is assumed to occur with 100% efficiency.
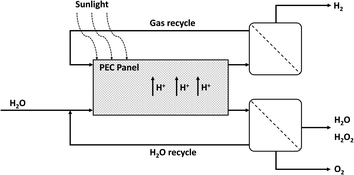 | ||
| Fig. 2 Schematic of the industrial PEC process with H2O2 being produced at the anode and H2 at the cathode. | ||
Techno-economic assumptions
Similar to other reports discussing the techno-economics of PEC water splitting, a large scale facility with a daily hydrogen production of 10 tonnes is considered.4,6,7 A summary of the technical assumptions is shown in Table 1. Importantly, we emphasize that many of the chosen parameters are dependent on the country and precise location of the PEC plant. Here, we have chosen parameters resembling input parameters of techno-economic studies focusing on ‘classic’ H2/O2 PEC water splitting. It is assumed that the PEC panel is placed in a suitable climate for water splitting. We further explore the dependence of the levelized cost of hydrogen on such parameters in a detailed sensitivity analysis. A solar energy input of 6.19 kW h m−2 d−1 resembling the average solar energy input measured for a 35° solar panel array tilted to the south in Daggett, California, USA is used.5–7,51 Because the facility will face downtime due to e.g. defects and maintenance, we introduce a capacity factor, i.e. a ratio between actual operation time and theoretically possible operation time. Because most of the maintenance can be done at night, a high capacity factor of 95% is used.5,51 Moreover, similar to earlier reports, we adapt an inflation rate of 1.9%,4,5,7,51 a tax rate of 38.9%,7 and a discount rate of 10%.7 Finally, we use the known price-range of H2O2 in 2006, which is $0.5–1.2 kg−1.8,20| Parameter | Value |
|---|---|
| H2 production scale4,6,7 | 10 t H2 per d |
| Solar energy input5–7,51 | 6.19 kW h m−2 d−1 |
| Starting year | 2020 |
| Project lifetime4,6,7 | 20 years |
| Replacement time4,5,51 | 7 years |
| Capacity factor5,51 | 95% |
| Inflation rate4,5,7,51 | 1.9% |
| Tax rate7 | 38.9% |
| Discount rate7 | 10% |
| H2O2 price range8,20 | $0.5–1.2 kg−1 |
For the analysis of capital expenditures (CAPEX), we distinguish between PEC cell module costs and costs related to the hard and soft balance of systems (BoS). The costs are highlighted in Table 2. Judging on previous studies,4,5,51 where configurations modeled for PEC water splitting to yield H2 with O2 as by-product are used, we estimate the base-case values of the dual photoabsorbers to be roughly $50 m−2. A price of $5 m−2, which resembles the price of a nickel–molybdenum mesh,5,51,52 is adopted for hydrogen evolution. The price of the anode catalyst is difficult to deduce due to the novelty of hydrogen peroxide evolution at an anode. In PEC water splitting, values of e.g. $0.10 m−2 or $1 m−2 have been used (for nickel),5,51,52 or $8 m−2 for the cathode and anode combined (using Pt and IrOx).4 In this study, we compensate for the uncertainties associated with anodic H2O2 production and assume an HPER anode price of $5 m−2. The price of the proton-exchange membrane is set at $50 m−2.4,5,51 For the housing, a value of $21 m−2 is adapted.5,51,52 The price for glass is set at $10 m−2,5,51,52 and the assembly costs at $20 m−2.5,51
| Costs | Value |
|---|---|
| CAPEX | |
| PEC cell module | |
| Dual photoabsorber (adapted from ref. 4, 5 and 51) | $50 m−2 |
| HER cathode5,51,52 | $5 m−2 |
| HPER anode | $5 m−2 |
| Membrane4,5,51 | $50 m−2 |
| Housing (adapted from ref. 5, 51 and 52) | $21 m−2 |
| Glass5,51,52 | $10 m−2 |
| Assembly5,51 | $20 m−2 |
| PEC module replacement costs 5,51 | |
| 75% after 7 years & 60% after 14 years | |
| Hard BoS | |
| H2 gas system5,51 | M$11.5 |
| H2O piping system (adapted from ref. 5 and 51) | M$2.6 |
| H2O2 piping system | M$2.6 |
| Electrolyte, H2O2 and H2O separator | M$5 |
| Process control system | M$6 |
| Soft BoS | |
| Installation costs5,51 | 20% of initial investment + replacement costs |
| Contingency costs5,51 | 30% of initial investment |
| Engineering & design costs5,51 | 5% of initial investment |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| OPEX | |
| Insurance5,7,51 | 2% of initial CAPEX of PEC module and hard BoS a−1 |
| Labor | M$4.5 a−1 |
We determine the hard and soft balance of system (BoS) costs based on previously defined values by Grimm and co-workers.5,51 We adapt the same H2 gas system of M$11.5, which includes piping, gas compressors, condensers and intercooling. Water management is installed for a total of M$1.3 for a PEC system where H2 and O2 are produced. We assume double costs for the piping due to double H2O quantities required for the production of 1 mole of H2 when H2O2 is produced at the anode (compare reactions (3) and (5)). Similarly, we included a H2O2 piping system worth M$2.6. Due to the uncertain nature of chemical separation, an additional penalty of M$5 was added for recycling of the electrolyte, as well as possible concentrating of the H2O2. Considering the process control system an expense of M$6 was assumed that is slightly larger than that for ‘normal’ PEC water splitting.5,51 In the CAPEX costs, ground costs ($0.15 m−2) are considered to be negligible.5,7,51
To estimate the operating expenditures (OPEX) costs, we set an insurance of 2% of the initial capital cost.5,7,51 For the labor costs, we assume that 4 security officers are available all day long at any time, thus spanning 96 man-hours each day. Similarly, we assume that daily 400 man-hours are required to maintain functionality of the system, for instance realized by installing two 8 hour shifts, with 25 employees working in each shift. With an average of $25 h−1,5,51 the annual labor costs can be roughly estimated to be M$4.5. Finally, the PEC cell modules will be replaced in 7 year intervals. The costs associated with panel replacement have been estimated to be 75% after 7 years and 60% after 14 years, in agreement with an expected decrease in production costs.5,51
Economic model used
The net present value (NPV) of a system describes its economic profitability.4,51 If the NPV < 0, a project will have a negative profitability potential, whereas NPV > 0 implies that the project is economically beneficial. The break-even point of a project, where the benefits and costs outweigh each other, is defined as NPV = 0. Thus, in this analysis, the minimum price at which H2 or H2O2 can be sold is calculated using NPV = 0. The NPV at the beginning of a year can be calculated using the following formula: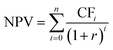 | (6) |
| CFi = Cashini − Cashouti − Taxi | (7) |
| Taxi = (Cashini − Cashouti − Depi)tratei | (8) |
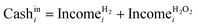 | (9) |
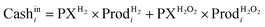 | (10) |
| Cashouti = CAPEXi + OPEXi | (11) |
In our model, we use a price range of $0.5 to 1.2 kg−1 for H2O2 (see Table 1).8,20 To achieve a net present value of 0, we calculate the corresponding costs at which the generated H2 needs to be sold, i.e. the levelized cost of hydrogen (LCH).4–7,51,52 To calculate the LCH, we use a flow chart as depicted in Fig. S3.† First, the required PEC panel area is calculated using the H2 production scale required, the faradaic efficiency towards H2O2 production, the solar-to-hydrogen (STH) efficiency (or simply H2 efficiency) and several fixed parameters, i.e. the Gibbs free energies involved in the electrochemical production of respectively H2O2 and O2 from water, the solar energy input, the capacity factor (95%, Table 1) and the molar mass of H2. For a detailed derivation of the formula, we refer to the ESI (eqn (S15)†). Using the calculated PEC panel area the total OPEX and CAPEX costs and depreciation are calculated. The computed area is furthermore used to calculate the annual production of H2O2 (see ESI, eqn (S20)†) and thus the income over the sale of H2O2. Taking into account that taxation is dependent on the CAPEX costs, the OPEX costs, the depreciation and the income over the sale of H2O2 and H2, the required income of H2 can be calculated to achieve a net present value (NPV) of 0. From here, the minimum price at which H2 needs to be sold is calculated in $ per kg.
Results and discussions
Scenario (i): near optimal scenario
A contour plot of the H2 price as a function of the solar-to-hydrogen (STH) efficiency and the H2O2 price is depicted in Fig. 3. A summary of the most important values derived from this figure is demonstrated in Table 3.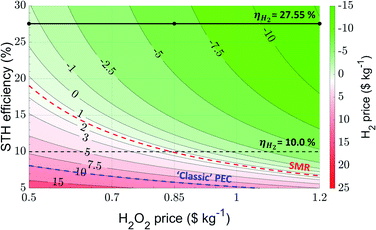 | ||
| Fig. 3 Contour plot demonstrating the H2 price as a function of the STH efficiency and H2O2 price using parameters defined in Tables 1 and 2 and with a faradaic efficiency of 100% for anodic H2O2 production. The black solid line implies the theoretical maximum of STH efficiency, the black dotted line an STH efficiency of 10%. The red dotted line corresponds to a H2 price of $1.4 kg−1, i.e. the price of hydrogen formed through steam methane reforming (SMR), whereas the blue dotted line demonstrates the approximate levelized cost of hydrogen (ca. $10 kg−1) obtained using ‘classic’ PEC water splitting, i.e. through the production of O2 at the anode. | ||
| H2O2 price ($ per kg) | STH efficiency (%) | LCH ($ per kg) |
|---|---|---|
| 0.5 | 8.11 | 10.0 |
| 10.0 | 7.18 | |
| 19.1 | 1.4 | |
| 27.55 | −0.560 | |
| 0.85 | 5.82 | 10.0 |
| 9.90 | 1.4 | |
| 10.0 | 1.28 | |
| 27.55 | −6.45 | |
| 1.2 | 4.54 | 10.0 |
| 6.69 | 1.4 | |
| 10.0 | −4.61 | |
| 27.55 | −12.3 |
When the H2O2 price is fixed at $0.85 kg−1 and an STH efficiency of 27.55% (black solid line) is assumed as predicted for an ideal PEC system (see calculations in the ESI†), the calculated H2 price is $6.45 kg−1. Furthermore, at H2O2 prices of $1.2 kg−1 the H2 price is $12.3 kg−1, and even at a H2O2 price of $0.5 kg−1, the H2 price is still $0.560 kg−1. Theoretically, these negative values would imply that to reach NPV = 0, hydrogen should be distributed while also spending additional cash. Practically, the negative values mean that there is spare room to allow for higher debits, or simply that hydrogen can be sold at a high profit. The LCH values reported here demonstrate that theoretically it is possible to photoelectrochemically produce H2 at a cathode and H2O2 at an anode, and sell the H2 at a price cheaper than $1.4 kg−1, i.e. the H2 price through steam methane reforming, indicated by a red dotted line in Fig. 3. Thus, above this line hydrogen production by PEC is favored over SMR, highlighting the general flexibility of the PEC H2/H2O2 process. Using the standard case value of $0.85 kg−1 H2O2, a solar-to-hydrogen efficiency of only 9.90% is required to compete with SMR, whereas at respectively H2O2 prices of $0.5 kg−1 and $1.2 kg−1 STH efficiencies of 19.1% and 6.69% allow for competition. Although these values are relatively high, especially the values with an STH efficiency lower than 10% are not unreasonable: recent studies on photoelectrode–photovoltaic (PEC–PV) tandem cells have already demonstrated that STH efficiencies in this order of magnitude can be achieved.53 Moreover, as stated above, techno-economic studies investigating ‘classic’ PEC water splitting use an STH efficiency of ca. 10% to achieve an LCH of ca. $10 kg−1.4,6,7 Here, our analysis of the H2/H2O2 PEC system predicts H2 prices of $7.18 kg−1, $1.28 kg−1 and $4.61 kg−1 for the H2O2 prices of $0.5 kg−1, $0.85 kg−1 and $1.2 kg−1 at an STH efficiency of 10% (black dotted line). In fact, to be competitive with ‘classic’ PEC water splitting (highlighted by the blue dotted line), STH efficiencies of only 8.11%, 5.82% and 4.54% are required, clearly highlighting the benefits of the H2/H2O2 PEC system. In a broader view, the trends demonstrated in this section also clarify the importance of producing a valuable product at the anode: the higher the price of the product, the easier it becomes to sell H2 at low prices.
Scenario (ii): current state-of-the-art scenario
Next, we proceed to reveal the techno-economics of a current state-of-the-art scenario, where recently published data are used as input parameters for the techno-economic model defined in this work. We use the pioneering work of Shi et al.,29 where BiVO4 coated on fluorine-doped tin oxide (FTO) was used as a photoanode (connected to a ‘dark’ cathode) for the production of H2O2 in a bicarbonate (NaHCO3) electrolyte. Under solar simulation, the authors demonstrate that faradaic efficiencies of ca. 98% at current densities of 5.7 mA cm−2 are obtained at an applied potential of 1.9 V vs. RHE. In our model, we use a hybrid PEC/PV-configuration where a photovoltaic module provides the additional voltage required to maintain the operating potential of the system, similar to studies by Fuku et al.26 In those studies, the required voltage was generated by double dye-sensitized solar cells (DSSCs) in combination with a BiVO4/WO3 photoanode. Based on previous studies,4,54,55 we assume that a PV cell can be optimized to yield a voltage of 1.9 V and a current of 5.7 mA cm−2. Using the latter value, we proceed to calculate the corresponding STH efficiency using formula (S3) (defined in the ESI†). Here, it is important to note that the potential difference deviates from the commonly used 1.23 V for water splitting into hydrogen and oxygen. When oxygen is substituted with hydrogen peroxide at the anode, the thermodynamic potential becomes 1.78 V. Considering a mixed production of hydrogen peroxide and oxygen with hydrogen peroxide significantly exceeding oxygen evolution, an STH efficiency of 10.1% is calculated. To calculate the LCH, we assume a photoanode price of $50 m−2, which corresponds roughly to semiconductor costs used in other techno-economic analyses.4,5,51 We have chosen to set the price per m2 of BiVO4 high compared with the market value56 due to uncertainties associated in the processing conditions of the photoanode. For the additional PV cell, we adapt a highly efficient multi-silicon module with a price of $0.215 W−1 and an efficiency of 18.8% as reported on EnergyTrend.57 With a solar input of roughly 1000 W m−2 and using the ratios between PV module cost, wiring costs and mounting costs reported earlier,58 we estimate the total additional costs of the PV module to be $70 m−2. It should be noted that these costs are higher than the dual photoabsorber costs used in Table 2. This makes sense, as the dual photoabsorbers are already integrated within an operating system. Thus, no additional wiring and mounting costs are taken into account for the latter. Finally, it is important to note that in this state-of-the-art design a different electrolyte is used, rendering separation of H2O2 probably slightly more complex. To facilitate separation in scenario (ii) a steam process will be used as well.16 Here, sodium bicarbonate will decompose in sodium carbonate, water and carbon dioxide.59 Thus, a vapor will be separated from the electrolyte consisting of H2O2, H2O and CO2. We propose recycling of CO2 to maintain the ‘greenness’ of the H2/H2O2 PEC process. Therefore, H2O2 is separated from the H2O and the CO2 using a fractionating column and subsequently H2O and CO2 are fed back to sodium carbonate solution ensuring formation of sodium bicarbonate. The resulting mixture is recycled to the PEC plant.A schematic depicting the new configuration is demonstrated in Fig. 4. An overview of the new specifications for the techno-economic analysis is given in Table 4.
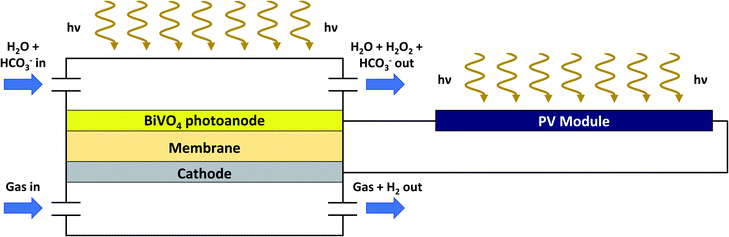 | ||
| Fig. 4 Hybrid PEC/PV-configuration for the production of H2 and H2O2 based on a current state-of-the-art scenario. Similar conditions as in Fig. 1 are used. In contrast, light absorption is governed both by a BiVO4 photoanode and a photovoltaic panel. Furthermore, bicarbonate (HCO3−) is introduced as an electrolyte at the anodic site. | ||
| Costs | Value |
|---|---|
| PV modules (adapted from ref. 57 and 58) | $70 m−2 |
| HPER BiVO4 photoanode (adapted from ref. 4, 5 and 51) | $50 m−2 |
Using the new set of input parameters for scenario (ii), we calculated the levelized cost of hydrogen as a function of H2O2 price and STH efficiency, shown in Fig. 5. Once more, the most important values derived from this figure are summarized in Table 5.
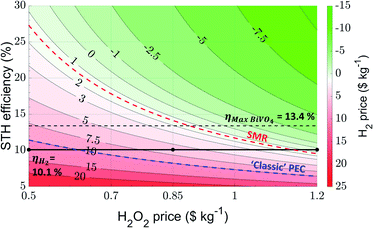 | ||
| Fig. 5 Contour plot demonstrating the H2 price as a function of the STH efficiency and H2O2 price based on a current state-of-the-art scenario, where a hybrid PEC/PV device configuration is used for the production of H2 and H2O2. The black line implicates the STH efficiency corresponding to the work by Shi et al. (10.1%).29 A black dotted line is used to elucidate the theoretical maximum STH value when BiVO4 is used as a photoanode for H2O2 production. The red and blue dotted lines correspond respectively to H2 prices of $1.4 kg−1 and $10 kg−1. The former resembles the price of H2 obtained through steam methane reforming (SMR), whereas the latter represents the approximate LCH obtained using ‘classic’ PEC water splitting. | ||
| H2O2 price ($ per kg) | STH efficiency (%) | LCH ($ per kg) |
|---|---|---|
| 0.5 | 10.1 | 12.0 |
| 11.4 | 10.0 | |
| 13.4 | 7.84 | |
| 27.3 | 1.4 | |
| 0.62 | 10.1 | 10.0 |
| 0.85 | 8.23 | 10.0 |
| 10.1 | 6.19 | |
| 13.4 | 2.06 | |
| 14.1 | 1.4 | |
| 0.89 | 13.4 | 1.4 |
| 1.14 | 10.1 | |
| 1.2 | 6.43 | 10.0 |
| 9.54 | 1.4 | |
| 10.1 | 0.415 | |
| 13.4 | −3.71 |
Obviously the contour plot reveals similar trends for the LCH as the contour plot predicted for scenario (i), i.e. the dual photoabsorber reactor without additional voltage supply (Fig. 3). However, in contrast to the calculations shown for scenario (i), a clear offset in the contour plot for scenario (ii) is observed, i.e. independent of the H2O2 price higher STH efficiencies must be achieved to allow for economically profitable H2 production. From Fig. 5, we derive an LCH value of $6.19 kg−1 when the STH efficiency is 10.1% and the hydrogen peroxide price is $0.85 kg−1. Although this is not yet on par with the LCH value of H2 produced using steam methane reforming, our analysis predicts that H2 can be produced at lower costs than via ‘classic’ PEC water splitting. Interestingly, a slight increase in STH efficiency, i.e. up to 14.1%, allows for H2 production being financially competitive with hydrogen produced through steam methane reforming (ca. $1.4 kg−1). However, it is important to contemplate that the theoretical maximum achievable photocurrent with BiVO4 is limited to 7.5 mA cm−2.60,61 For anodic H2O2 production and cathodic H2 production (with 100% faradaic efficiencies for both reactions) the maximum achievable STH efficiency is 13.4% (using formula (S1)† and a thermodynamic potential difference of 1.78 V). To achieve higher STH efficiencies (such as 14.1%), replacement of the BiVO4 with a lower bandgap photoanode is necessary. Still, for highly efficient BiVO4, i.e. at maximum STH efficiency of 13.4%, the calculated LCH is only $2.06 kg−1, nearing competition with steam methane reforming very closely.
An alternative strategy is to increase the H2O2 price to approximately $0.89 kg−1 or $1.14 kg−1 at an STH efficiency of respectively 13.4% or 10.1%. This yields an LCH competitive to steam methane reforming as well. Even further increasing the H2O2 price leads to even lower H2 prices, e.g. $0.415 kg−1 at a H2O2 price of $1.2 kg−1. At this H2O2 price, an STH efficiency of 9.54% is required to compete with steam methane reforming. A low H2O2 price of $0.5 kg−1 yields a high LCH of $12.0 kg−1 (at an STH efficiency of 10.1%), which is clearly not advantageous anymore over ‘classic’ PEC water splitting. Nevertheless, it is important to realize that highly optimized systems have been used to predict the LCH of ‘classic’ PEC water splitting, whereas here a state-of-the-art system has been considered. Still, a very high STH efficiency of 27.3% is needed to compete with steam methane reforming at such low H2O2 prices. Clearly, the H2 price is very dependent on the H2O2 price. Therefore, it is important that H2O2 is sold at a sufficiently high price to make a PEC(/PV) system for H2 and H2O2 production economically more attractive than SMR. Our calculations nicely reveal that a PEC(/PV) system for H2 and H2O2 production can easily compete with ‘classic’ PEC water splitting.
Cost comparison and sensitivity analysis
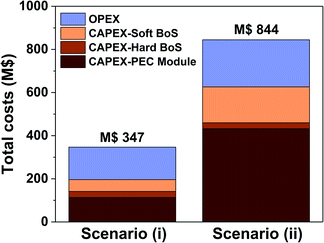
As described in the methodology section, many input parameters were defined on the basis of previous techno-economic studies investigating ‘classic’ H2/O2 PEC water splitting. However, it is important to realize that many of these input parameters are variable and could depend on e.g. the country or the location where the H2/H2O2 PEC plant is situated. Therefore, we proceed to perform a sensitivity analysis to elaborate the dependency of the LCH on various input parameters. To understand the sensitivity of our model on the predicted LCH values, we first perform a thorough analysis of the CAPEX and OPEX costs, followed by a cost variation of individual parts of the system. In Fig. 6 the magnitude of the total CAPEX and the OPEX costs during the 20 year operation of the H2/H2O2 PEC plant for both scenario (i) and (ii) are shown. Clearly, and as expected, more expenses are involved in the current state-of-the-art scenario than in the near-optimal scenario. Especially the CAPEX costs of the PEC cell and of the soft BoS are larger, but to some extent the OPEX costs as well. The hard BoS costs remain identical. The huge increase in the CAPEX costs of the PEC cell module is due to the significant increase in materials costs: the dual photoabsorbers and the HPER anode, with a total cost of $55 m−2, have been replaced by costs for both the PV modules and the BiVO4 photoanode with a total cost of $120 m−2. The dependency of the soft BoS and the OPEX costs on the PEC module costs explains the increase in those debits. It is worth noting that for the near-optimal scenario, the total CAPEX and OPEX costs are in the same order of magnitude (M$196 vs. M$150 respectively), whereas for the current state-of-the-art scenario, the CAPEX costs clearly outweigh the estimated OPEX costs (M$626 vs. M$218 respectively).To elucidate further on the dependency of the H2 price as a function of input parameters, we proceed by performing a sensitivity analysis for the current state-of-the-art scenario described in this work, since such a H2/H2O2 PEC plant will be closer to implementation at this moment than one based on the near-optimal scenario. A base-case H2O2 price of $0.85 kg−1 is used. The results of the sensitivity analysis are summarized in Fig. 7.
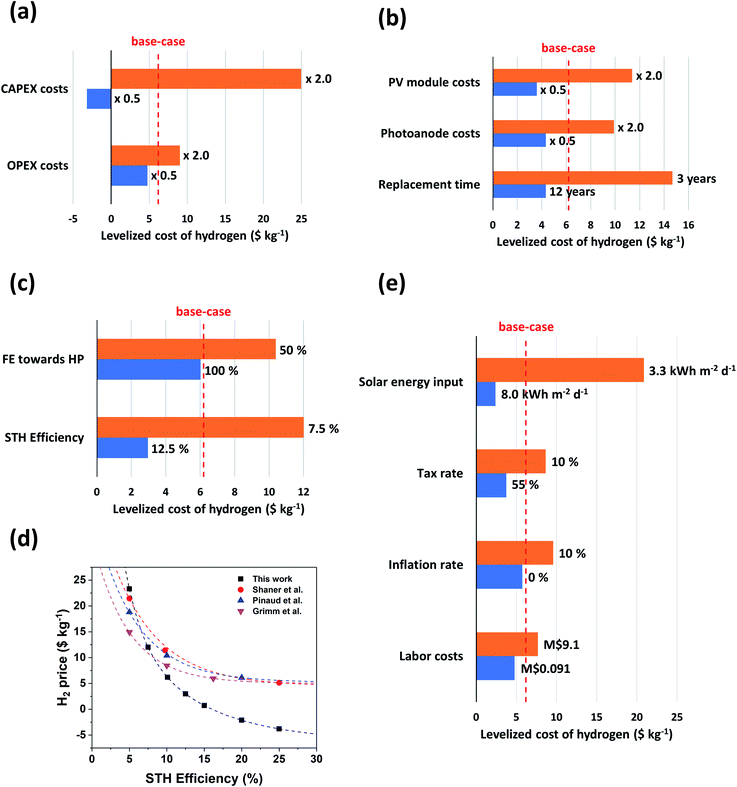 | ||
| Fig. 7 Sensitivity analysis for the current state-of-the-art scenario of a H2/H2O2 PEC plant. The LCH is plotted as a function of (a) CAPEX and OPEX costs; (b) PV module costs, photoanode costs and replacement time; (c) faradaic efficiency towards hydrogen peroxide (HP) production and STH efficiency and (e) solar energy input, tax rate, inflation rate and labor costs. The red dotted line indicates the base-case LCH value, i.e. $6.19 kg−1 H2 at a H2O2 price of $0.85 kg−1, a faradaic efficiency of 98% an STH efficiency of 10.1% and a replacement time of 7 years. The base-case values of (e) are defined in Tables 1 and 2. (d) Comparison of the LCH as a function of STH efficiencies in this work and other works from literature.4–6 | ||
In Fig. 7a, we first highlight the difference on the influence of the CAPEX and OPEX costs on the LCH value when there is an increase of 100% or a decrease of 50%. Clearly, the influence of the CAPEX is much larger than the OPEX. This is expected, considering that the CAPEX costs make up the majority of the financial expenses for the current state-of-the-art scenario (see Fig. 6). A major change in the OPEX costs doesn't have significant implications on the LCH. Practically, this could imply that more personnel or larger wages can easily be considered to allow for a more smoothly running H2/H2O2 PEC plant. Changes in the CAPEX costs on the other hand have quite a dramatic influence on the LCH: a reduction of 50% in the CAPEX costs reduces the LCH price from $6.19 kg−1 to $3.19 kg−1. On the other hand, an increase of 100% in CAPEX yields a hydrogen price of $25.0 kg−1.
Clearly, the LCH sensitivity on the CAPEX is huge, and therefore we proceed to break down those CAPEX costs (Fig. 7b). To do so, the costs of the PV module and the photoanode, both large contributors in the total price, and the replacement time of the PEC module are evaluated. For the PV module, we assumed a total cost of $70 m−2, where mounting materials and wiring costs are included.57,58 However, Shaner et al. adopt a much larger total PV price of $141 m−2,4 which would yield a H2 price of $11.5 kg−1 in our study. For the photovoltaic module costs, the authors adopt prices for non-subsidized, single crystalline Si PV modules from the year 2015, whereas we adopt prices of a highly efficient multi-silicon module in 2019 from the same database (i.e. EnergyTrend).57 Clearly, prices of PV modules are dropping. Therefore, it is also realistic to assume that in the future, the prices might even be lower. If the price of the PV module is halved, the LCH is only $3.60 kg−1, a considerable improvement compared to the LCH of $6.19 kg−1 predicted for the base-case of scenario (ii). As expected, sensitivity analysis for the photoanode costs shows similar trends as for the PV module costs. In this work, we estimate the cost to be $50 m−2.4,5,51 However, because of the novelty of anodic hydrogen peroxide production, little is known yet about the material best suited for anodic H2O2 production. Correspondingly the materials price is hard to estimate. If the photoanode cost is doubled, an LCH of $9.90 kg−1 is calculated. However, a reduction of the cost is rewarding: half the price of the photoanode cost would yield an LCH of $4.34 kg−1. The price range for the PV module and photoanode indicates that industrial development of large scale H2/H2O2 PEC systems can be attainable in the near future. Another important feature is the durability of the PEC module. In fact, if the PEC module has to be replaced frequently, the consequences for the LCH are significant. When the PEC module is replaced already after every 3 years, the hydrogen price more than doubles ($14.7 kg−1). When replacement is done every 12 years (practically meaning that replacement is done only once in the 20 years of operation), the LCH would be $4.33 kg−1. Clearly, material stability is one of the most important factors that needs to be considered for a profitable H2/H2O2 PEC plant. Therefore improvement of the durability of the HPER electrode should be stimulated.
Moreover, we study the influence of the faradaic efficiency (FE) to H2O2 production and the solar-to-hydrogen (STH) efficiency of the system (Fig. 7c). As indicated in eqn (S3),† the STH efficiency is amongst others dependent on the FE towards H2O2. In the current state-of-the-art scenario, the FE for H2O2 was almost 100%. Care should be taken to have sufficient FE; reduction of the FE to 50% for instance would imply an increase of the LCH to $10.4 kg−1. In fact, a faradaic efficiency of 0% to H2O2 production would mean a faradaic efficiency of 100% to O2 production. Thus, the PEC system becomes a ‘classic’ PEC water splitting system. In such a case, the LCH would be $14.8 kg−1. This value is slightly higher than LCH values for a ‘classic’ H2/O2 PEC system reported in literature at an STH efficiency of 10% (ca. $10 kg−1).4,6,7
The influence of the STH efficiency on the LCH is much larger in our model. An increase of the STH efficiency from 10.1% to 12.5% yields an astonishing LCH drop to $2.97 kg−1. Similarly, a decrease in STH efficiency to 7.5% yields an LCH value of $12.0 kg−1. In previous studies on the techno-economics of ‘classic’ PEC water splitting, the high dependency of the hydrogen price on STH efficiency has also been reported.4–6 In Fig. 7d, we compare the dependency of the H2 price as a function of the STH efficiency in our work with the values reported in those studies. Clearly, we predict that this dependency is more extreme for a H2/H2O2 PEC system than for a H2/O2 PEC system. This also means that an identical change in STH efficiency is more rewarding for the H2/H2O2 PEC system. At STH efficiencies higher than 8.3%, the H2/H2O2 PEC system yields lower LCH values than the best performing H2/O2 PEC system reported by Grimm et al.5 Thus, selective water oxidation to H2O2 over O2 is more rewarding at those STH efficiencies. Clearly, controlling the STH efficiency is a crucial factor in achieving profitable H2/H2O2 PEC plants. As the FE towards H2O2 is already close to 100%, other aspects need to be improved to attain higher STH efficiencies. The only non-fixed variable which allows for this is the operating current density (see eqn (S3)†). Hence, researchers should look for ways to increase the current density while maintaining similar faradaic efficiencies, e.g. by optimizing the BiVO4 photoanode or development of highly efficient anodes with smaller bandgap, similar to the materials development strategies used for ‘classic’ PEC water splitting devices.
Finally, we investigate the dependency of the LCH on parameters influenced by the location and country of the H2/H2O2 PEC plant, specifically the solar energy input, the tax rate, the inflation rate and the labor costs. The results are summarized in Fig. 7e. For the solar energy input, we base a negative scenario on the solar energy input in Enschede, The Netherlands; for a positive scenario the Atacama Desert in northern Chile was chosen. With optimal tilt of the panels, the solar energy inputs are respectively 3.3 kW h m−2 d−1 and ca. 8.0 kW h m−2 d−1.62 These inputs yield LCH values of respectively $20.9 kg−1 and $2.40 kg−1, revealing the significant dependence of the LCH on the location of the H2/H2O2 PEC plant. Thus, it is vital to locate the plant in a dry and sunny area, such as California, the Atacama Desert, Australia, the Arabian Desert or the Sahara Desert in Africa. This is of course also true for ‘classic’ PEC water splitting devices. The influence of the tax rate is considerably less: an increase in tax rate to 55% (possible in the United Arab Emirates) yields an LCH of $8.66 kg−1, whereas using a tax rate of 10% (used in e.g. Qatar) results in an LCH of $3.76 kg−1.63 Similarly, the influence of the inflation rate is also not high. Increasing the inflation to a high value of 10% would yield a hydrogen price of $9.59 kg−1, whereas no inflation would imply a hydrogen price of $5.76 kg−1. Finally, we proceed to break down labor costs. Based on averaged incomes, we roughly estimate that a high salary for employment in the H2/H2O2 PEC plant could be $50 h−1 and a low salary could be $0.50 h−1 (for instance possible when the plant would be located in Switzerland or Ethiopia, respectively).64 This would correspond roughly with annual labor costs of M$9.1 a−1 and M$0.091 a−1. Implementation in our sensitivity analysis yields values of $7.68 kg−1 H2 and $4.76 kg−1 H2, rendering the consequence of labor costs for the predicted H2 price small. This result is in line with the prediction that the sensitivity of the LCH on the OPEX costs is significantly lower than the sensitivity of the LCH on the CAPEX costs.
Concluding, our sensitivity analysis of the current state-of-the-art scenario predicts that the design and implementation of a H2/H2O2 PEC system approaches financial feasibility. For the base-case scenario, the LCH is already advantageous over a ‘classic’ PEC water splitting system. Even when a negative scenario for a variable is assumed (with the exception of the total CAPEX costs and the solar energy input), the LCH value is close to or is even still lower than the H2 price predicted for ‘classic’ PEC water splitting devices. Similarly, when an optimistic scenario is assumed, possible competition of a H2/H2O2 PEC system with steam methane reforming draws nearer. Specifically, judging by the strong dependency of the LCH, an increase in the STH efficiency seems critical. Materials stability also seems a key factor: while an increase in lifetime only yields a limited reduction of the H2 price, a decrease has detrimental effects. Finally, by far the most defining parameter for the choice of location and country would be the solar energy input. It is recommended to install the PEC plant in a sun-drenched environment.
Discussion
In this work, we clearly demonstrated the high potential of reducing the hydrogen price by selective photoelectrochemical water splitting with hydrogen peroxide production at the anode. In fact, when the market value of H2O2 and the STH efficiency is sufficiently high, a H2/H2O2 PEC configuration will be competitive with hydrogen production obtained through steam methane reforming. In this manuscript, we assumed a H2O2 price range of $0.5 kg−1 to $1.2 kg−1. However, the data used to estimate the H2O2 price are from 2006, and thus the H2O2 market value is expected to be higher in the current year 2020. Indeed, the price of 50% H2O2 on Kemcore is $0.75 kg−1.65 In such case, the price for pure 100% H2O2 would be $1.5 kg−1. Fig. 3 and 5 clearly demonstrate that a higher H2O2 price is advantageous for achieving a lower H2 price. To be competitive with hydrogen produced from steam methane reforming, this would also mean that a lower solar-to-hydrogen efficiency is needed. Thus, it would be easier to actually achieve this competitiveness.In the sensitivity analysis we predicted that a H2/H2O2 PEC system is close to financial feasibility. We have demonstrated that, aside from the location of the H2/H2O2 PEC plant, especially the improvement of the STH efficiency is important to achieve competitiveness with steam methane reforming. Furthermore, it is important that stable materials are used. Research on anodic materials for hydrogen peroxide production is still very novel. Consequently, there is plenty of opportunity to improve materials on solar-to-hydrogen and solar-to-hydrogen peroxide efficiencies, as well as on stability. In our model, for the state-of-the-art scenario, we assumed that BiVO4 is stable. However, later studies by Baek et al. have demonstrated that the stability of BiVO4 during H2O2 production is insufficient.38 Strategies to overcome this could be the addition of a protective layer (e.g. Jeon et al. use phosphate treatment on Mo:BiVO4),39 or to dope the material with a stabilizing agent (Baek et al. use gadolinium (Gd) for instance).38 Still, we chose to use BiVO4 as a model in this study, as we believe that stable anodes with a similar price range and with similar photoelectrochemical properties can be engineered in the future. Alternatively, rather than employing a photoelectrochemical cell for hydrogen and hydrogen peroxide production, a photovoltaic-electrolytic system can be used. Here, an optimized anode for the production of hydrogen peroxide needs to be found.
An important feature of H2/H2O2 PEC systems on the industrial scale is that hydrogen peroxide can be produced on-site, as opposed to the anthraquinone process.11,12,14,15,17,18 This is a big advantage, considering that hydrogen peroxide is a hazardous chemical and is very prone to decomposition when trace amounts of catalyst (such as metal ions) are present.16,19 Prolonged transport of hydrogen peroxide with the addition of a stabilizer is thus not required anymore. Still, it is good to keep in mind that even with on-site production, conditions for the fast decomposition of H2O2 should be avoided. H2O2 is significantly unstable in basic conditions, particularly when the pH is larger than 9.11,19 Qiang et al. also reported some degree of H2O2 decomposition around a pH-value of 3, possibly due to the trace presence of ferrous iron. Therefore, we advise to perform H2O2 generation in (preferably strong) acidic conditions. Here, in scenario (ii), we made use of a bicarbonate (HCO3−)-containing solution. Typically, bicarbonate acts as a buffer through an equilibrium with carbonic acid (CO2 + H2O, pKa = 6.4) and carbonate (CO32−, pKa = 10.3).24,66,67 A fresh bicarbonate solution without any pH adjustment will have a pH around 8. This would be in the ‘safe’ pH-range of H2O2 stability. Still, lowering of the pH through CO2 purging could be considered. Alternatively, the bicarbonate could be substituted with an electrolyte with strong acidic properties. For instance, judging by its usage in history for H2O2 production, sulfuric acid could be a potential candidate.11,16,21–23 Furthermore, it is also important that the H2O2 is not exposed to temperatures higher than room temperature to prevent decomposition.19 Therefore, cooling down of the H2O2 after synthesis might be rewarding as well.
In the production of an industrial H2/H2O2 PEC system, it is very important that the energy required to build the plant does not exceed the chemical energy harvested. In literature, this concept is referred to as the renewable energy factor (REF),68,69 or the energy return on energy invested (EROEI).70,71 The EROEI can be calculated using the following formula:
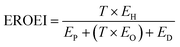 | (12) |
To increase the EROEI value (and thus the ‘greenness’ of the H2/H2O2 PEC plant) even further to well above 1, a strategy would be to concentrate solar energy on the PEC module (and, if used, the additional PV module). A tracking concentrator array could be used to achieve such light concentration.4,6,7 Typically, a parabolic cylinder array is used to focus solar light on a (linear) PEC module and a tracking system is used to align the concentrator array for optimal solar illumination harvesting during the day. Such devices can concentrate solar illumination to a factor of 10. As demonstrated in the sensitivity analysis in Fig. 7e, increasing the light intensity per m2 would also result in a steep decrease of the LCH. Larger concentration factors are possible, but care should be taken that currents larger than 1 A cm−2 are avoided. This is due to catalyst limitations, bubble formation (which scatter light) and temperature constraints.6,72
In a broader context, we have demonstrated in this study that (photo)electrochemical hydrogen evolution becomes more interesting when a valuable product at the anode is formed. Although sustainable H2 production at the TW level can only be achieved through water splitting, co-(photo)electrolysis might facilitate market penetration of the required PEC systems. Particularly, we have demonstrated that scenario (ii) already nears competition with steam methane reforming. Besides H2O2, chlorine gas (Cl2), bromine gas (Br2) and sodium hydroxide (NaOH) are usually advocated as interesting anodic products.8,54 More recently, Palmer et al. showed that also fluorine, iodine, methane decomposition (to carbon), potassium permanganate, sodium bromate, sodium chlorate and sodium persulfate production should be considered.46 They based this on the calculation of the net value of the chemicals per unit of energy input. Although in this study a black box approach has been used without taking CAPEX and OPEX costs into account, it still gives a good overview on which commodity chemicals might be interesting to be produced by means of a photoelectrochemical system. It should be mentioned that the study of Palmer et al. also demonstrates that substitutes for hydrogen at the cathode, for example tellurium, cobalt or tungsten, could be interesting to synthesize through (photo)electrochemical means while producing hydrogen peroxide at the anode. Alternatively, the (photo)electrochemical reduction of carbon dioxide with concomitant hydrogen peroxide should be considered too.73 Moreover, it was also recently demonstrated that hydrogen peroxide could be produced at both the anode and the cathode from selective water oxidation and oxygen reduction respectively.26,28,39
When we use the method of Palmer et al. to calculate the net value per unit of energy input for H2O2 (with hydrogen at the cathode),46 we find a value of $0.30 kW h−1 when the H2O2 price is $0.85 kg−1. In comparison, the anodic synthesis of bromine, iodine and sodium bromate, as well as the anodic decomposition of methane to carbon, yield a higher maximum net value per energy and are thermodynamically more favorable. Therefore, they could be worthwhile of investigation as well. Still, the market size of hydrogen peroxide is larger than for those chemicals (with the exception of methane decomposition to carbon),13,46 implying that hydrogen production with concomitant hydrogen peroxide production is still one of the most rewarding approaches to achieve industrial implementation of PEC water splitting.
Conclusions
In this work, we performed a techno-economic analysis to investigate the feasibility of anodic hydrogen peroxide production as a substitute for oxygen to decrease the levelized cost of hydrogen (LCH) in photoelectrochemical (PEC) water splitting. Using a near-optimal scenario, only an STH efficiency of 9.90% is needed to compete with steam methane reforming at a H2O2 price of $0.85 kg−1. For a current state-of-the-art scenario with an STH efficiency of 10.1% and also a H2O2 price of $0.85 kg−1, an LCH of $6.19 kg−1 was calculated, an obvious improvement compared to LCH values found in ‘classic’ water splitting. Clearly, this study demonstrates the financial advantages of replacing oxygen at the anode in PEC water splitting with hydrogen peroxide. Therefore, further research of photoelectrochemical H2O2 production at the anode should be stimulated. A sensitivity analysis on the current state-of-the-art scenario demonstrates that reduction of the CAPEX costs will contribute to allow H2/H2O2 PEC systems (here connected to additional photovoltaic modules) to be financially competitive with hydrogen formation through steam methane reforming. Key factors in reducing this cost will be the improvement of the STH efficiency, optimization of stability and choosing a sunlit location for the H2/H2O2 PEC plant. Research on novel materials for hydrogen peroxide production should be stimulated, while simultaneously the importance of the stability should not be neglected. Once the anode material has been properly engineered, anodic H2O2 production could have a promising future in industry for hydrogen production through (photo-)electrochemical means. In a broader context, we have demonstrated that the production of a valuable commodity chemical at an anode could play a key role for obtaining LCH's in PEC water splitting competitive with the LCH's in steam methane reforming.Conflicts of interest
There are no conflicts to declare.Abbreviations
| BoS | Balance of systems |
| CAPEX | Capital expenditures |
| DSSCs | Dye-sensitized solar cells |
| EROEI | Energy return on energy invested |
| FE | Faradaic efficiency |
| FTO | Fluorine-doped tin oxide |
| HER | Hydrogen evolution reaction |
| HP | Hydrogen peroxide |
| HPER | Hydrogen peroxide evolution reaction |
| LCH | Levelized cost of hydrogen |
| NPV | Net present value |
| OPEX | Operating expenditures |
| PEC | Photoelectrochemical |
| PV | Photovoltaic(s) |
| PV-E | Photovoltaic-electrolysis |
| REF | Renewable energy factor |
| SMR | Steam methane reforming |
| STH efficiency | Solar-to-hydrogen efficiency |
| STHP efficiency | Solar-to-hydrogen peroxide efficiency |
| WBM | Web-based model |
Acknowledgements
The authors are grateful to Mats Wildlock and Nina Simic from Nouryon (Bohus, Sweden) for fruitful discussions. Also, we would like to thank the Topconsortium voor Kennis- en Innovatie Biobased Economy (TKI-BBE) (TKI-BBE 1803), the Topconsortium voor Kennis- en Innovatie Chemie (TKI Chemie) (Chemie.PGT.2019.007) and Nouryon for financial support. Alexa Grimm is acknowledging financial support by Shell Global Solutions.References
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- M. R. Shaner, H. A. Atwater, N. S. Lewis and E. W. McFarland, Energy Environ. Sci., 2016, 9, 2354–2371 RSC.
- A. Grimm, W. A. De Jong and G. J. Kramer, Int. J. Hydrogen Energy, 2020 Search PubMed , submitted.
- B. A. Pinaud, J. D. Benck, L. C. Seitz, A. J. Forman, Z. Chen, T. G. Deutsch, B. D. James, K. N. Baum, G. N. Baum, S. Ardo, H. Wang, E. Miller and T. F. Jaramillo, Energy Environ. Sci., 2013, 6, 1983–2002 RSC.
- B. D. James, G. N. Baum, J. Perez and K. N. Baum, Technoeconomic analysis of photoelectrochemical (PEC) hydrogen production, Directed Technologies Inc., Arlington, Virginia, 2009 Search PubMed.
- B. Mei, G. Mul and B. Seger, Adv. Sustainable Syst., 2017, 1, 1600035 CrossRef.
- J. Joy, J. Mathew and S. C. George, Int. J. Hydrogen Energy, 2018, 43, 4804–4817 CrossRef CAS.
- J. Chen, D. Yang, D. Song, J. Jiang, A. Ma, M. Z. Hu and C. Ni, J. Power Sources, 2015, 280, 649–666 CrossRef CAS.
- S. Yang, A. Verdaguer-Casadevall, L. Arnarson, L. Silvioli, V. Čolić, R. Frydendal, J. Rossmeisl, I. Chorkendorff and I. E. L. Stephens, ACS Catal., 2018, 8, 4064–4081 CrossRef CAS.
- J. M. Campos-Martin, G. Blanco-Brieva and J. L. G. Fierro, Angew. Chem., Int. Ed., 2006, 45, 6962–6984 CrossRef CAS PubMed.
- R. Ciriminna, L. Albanese, F. Meneguzzo and M. Pagliaro, ChemSusChem, 2016, 9, 3374–3381 CrossRef CAS PubMed.
- Y. Jiang, P. Ni, C. Chen, Y. Lu, P. Yang, B. Kong, A. Fisher and X. Wang, Adv. Energy Mater., 2018, 8, 1801909 CrossRef.
- S. Fukuzumi, Y. Yamada and K. D. Karlin, Electrochim. Acta, 2012, 82, 493–511 CrossRef CAS PubMed.
- G. Goor, J. Glenneberg, S. Jacobi, J. Dadabhoy and E. Candido, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2019, pp. 1–40 Search PubMed.
- C. Samanta, Appl. Catal., A, 2008, 350, 133–149 CrossRef CAS.
- K. Sayama, ACS Energy Lett., 2018, 3, 1093–1101 CrossRef CAS.
- Z. Qiang, J.-H. Chang and C.-P. Huang, Water Res., 2002, 36, 85–94 CrossRef CAS PubMed.
- ICIS, www.icis.com/chemicals, accessed Jan 2017.
- S. Ranganathan and V. Sieber, Catalysts, 2018, 8, 379 CrossRef.
- H. Meidinger, Liebigs Ann., 1853, 88, 57–81 CrossRef.
- H. Berthelot, C. R. Acad. Sci., 1878, 86, 71–76 Search PubMed.
- K. Fuku and K. Sayama, Chem. Commun., 2016, 52, 5406–5409 RSC.
- K. Fuku, Y. Miyase, Y. Miseki, T. Gunji and K. Sayama, ChemistrySelect, 2016, 1, 5721–5726 CrossRef CAS.
- K. Fuku, Y. Miyase, Y. Miseki, T. Funaki, T. Gunji and K. Sayama, Chem.–Asian J., 2017, 12, 1111–1119 CrossRef CAS PubMed.
- K. Fuku, Y. Miyase, Y. Miseki, T. Gunji and K. Sayama, RSC Adv., 2017, 7, 47619–47623 RSC.
- X. Shi, Y. Zhang, S. Siahrostami and X. Zheng, Adv. Energy Mater., 2018, 8, 1801158 CrossRef.
- X. Shi, S. Siahrostami, G.-L. Li, Y. Zhang, P. Chakthranont, F. Studt, T. F. Jaramillo, X. Zheng and J. K. Nørskov, Nat. Commun., 2017, 8, 701 CrossRef PubMed.
- Y. Miyase, S. Takasugi, S. Iguchi, Y. Miseki, T. Gunji, K. Sasaki, E. Fujita and K. Sayama, Sustainable Energy Fuels, 2018, 2, 1621–1629 RSC.
- A. Izgorodin, E. Izgorodina and D. R. MacFarlane, Energy Environ. Sci., 2012, 5, 9496–9501 RSC.
- C. McDonnell-Worth and D. R. MacFarlane, RSC Adv., 2014, 4, 30551–30557 RSC.
- S. Siahrostami, G.-L. Li, V. Viswanathan and J. K. Nørskov, J. Phys. Chem. Lett., 2017, 8, 1157–1160 CrossRef CAS PubMed.
- V. Viswanathan, H. A. Hansen and J. K. Nørskov, J. Phys. Chem. Lett., 2015, 6, 4224–4228 CrossRef CAS PubMed.
- Y. Ando and T. Tanaka, Int. J. Hydrogen Energy, 2004, 29, 1349–1354 CrossRef CAS.
- A. Naldoni, T. Montini, F. Malara, M. M. Mróz, A. Beltram, T. Virgili, C. L. Boldrini, M. Marelli, I. Romero-Ocaña, J. J. Delgado, V. Dal Santo and P. Fornasiero, ACS Catal., 2017, 7, 1270–1278 CrossRef CAS.
- Z. Han, K. T. Horak, H. B. Lee and T. Agapie, J. Am. Chem. Soc., 2017, 139, 9108–9111 CrossRef CAS PubMed.
- J. H. Baek, T. M. Gill, H. Abroshan, S. Park, X. Shi, J. Nørskov, H. S. Jung, S. Siahrostami and X. Zheng, ACS Energy Lett., 2019, 4, 720–728 CrossRef CAS.
- T. Jeon, H. Kim, H.-i. Kim and W. Choi, Energy Environ. Sci., 2020 10.1039/c9ee03154e.
- S. R. Kelly, X. Shi, S. Back, L. Vallez, S. Y. Park, S. Siahrostami, X. Zheng and J. K. Nørskov, ACS Catal., 2019, 9, 4593–4599 CrossRef CAS.
- S. Y. Park, H. Abroshan, X. Shi, H. S. Jung, S. Siahrostami and X. Zheng, ACS Energy Lett., 2019, 4, 352–357 CrossRef CAS.
- T. Shiragami, H. Nakamura, J. Matsumoto, M. Yasuda, Y. Suzuri, H. Tachibana and H. Inoue, J. Photochem. Photobiol., A, 2015, 313, 131–136 CrossRef CAS.
- F. Kuttassery, S. Mathew, S. Sagawa, S. N. Remello, A. Thomas, D. Yamamoto, S. Onuki, Y. Nabetani, H. Tachibana and H. Inoue, ChemSusChem, 2017, 10, 1909–1915 CrossRef CAS PubMed.
- S. Fukuzumi, Y.-M. Lee and W. Nam, Chem.–Eur. J., 2018, 24, 5016–5031 CrossRef CAS PubMed.
- J. Liu, Y. Zou, B. Jin, K. Zhang and J. H. Park, ACS Energy Lett., 2019, 4, 3018–3027 CrossRef CAS.
- C. Palmer, F. Saadi and E. W. McFarland, ACS Sustainable Chem. Eng., 2018, 6, 7003–7009 CrossRef CAS.
- B. Seger, O. Hansen and P. C. K. Vesborg, Sol. RRL, 2017, 1, e201600013 CrossRef.
- K. V. Titova, V. P. Nikol'skaya, V. V. Buyanov and I. P. Suprun, Russ. J. Appl. Chem., 2002, 75, 1903–1906 CrossRef CAS.
- A. E. Simpson and C. A. Buckley, Desalination, 1988, 70, 431–442 CrossRef CAS.
- J. M. Stinson and C. E. Mumma, Ind. Eng. Chem., 1954, 46, 453–457 CrossRef CAS.
- W. A. De Jong, Master thesis, Utrecht University, 2018.
- M. Victoria, PhD thesis, Delft University of Technology, 2015.
- J. H. Kim, D. Hansora, P. Sharma, J.-W. Jang and J. S. Lee, Chem. Soc. Rev., 2019, 48, 1908–1971 RSC.
- E. Chinello, M. A. Modestino, J. W. Schüttauf, L. Coulot, M. Ackermann, F. Gerlich, A. Faes, D. Psaltis and C. Moser, RSC Adv., 2019, 9, 14432–14442 RSC.
- S. Tembhurne and S. Haussener, Sustainable Energy Fuels, 2019, 3, 1297–1306 CAS.
- Kremer Pigmente; Bismuth-Vanadate Yellow, lemon, https://www.kremer-pigmente.com/en/1422/bismuth-vanadate-yellow-lemon, accessed Mar 2020 Search PubMed.
- EnergyTrend, https://www.energytrend.com/solar-price.html, accessed Nov 2019.
- International Technology Roadmap for Photovoltaic (ITRPV) - Results 2018 including maturity report 2019, October 2019 Search PubMed.
- C. Thieme, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2019, pp. 299–317 Search PubMed.
- F. F. Abdi, N. Firet and R. van de Krol, ChemCatChem, 2013, 5, 490–496 CrossRef CAS.
- P. Luan and J. Zhang, ChemElectroChem, 2019, 6, 3227–3243 CrossRef CAS.
- Global Solar Atlas, https://globalsolaratlas.info/map, accessed Mar 2020 Search PubMed.
- D. Bunn, Tax Foundation: Corporate Tax Rates Around the World, 2018, https://taxfoundation.org/corporate-tax-rates-around-world-2018, accessed Mar 2020 Search PubMed.
- WorldData.info: average income around the world, https://www.worlddata.info/average-income.php, accessed Mar 2020 Search PubMed.
- Kemcore, https://www.kemcore.com/hydrogen-peroxide-50.html, accessed Feb 2020.
- G. Czapski, S. V. Lymar and H. A. Schwarz, J. Phys. Chem. A, 1999, 103, 3447–3450 CrossRef CAS.
- W. W. Rudolph, G. Irmer and E. Königsberger, Dalton Trans., 2008, 900–908 RSC.
- F. Kuttassery, S. Mathew, S. N. Remello, A. Thomas, K. Sano, Y. Ohsaki, Y. Nabetani, H. Tachibana and H. Inoue, Coord. Chem. Rev., 2018, 377, 64–72 CrossRef CAS.
- F. Kuttassery, D. Yamamoto, S. Mathew, S. N. Remello, A. Thomas, Y. Nabetani, A. Iwase, A. Kudo, H. Tachibana and H. Inoue, J. Photochem. Photobiol., A, 2018, 358, 386–394 CrossRef CAS.
- R. Sathre, C. D. Scown, W. R. Morrow, J. C. Stevens, I. D. Sharp, J. W. Ager, K. Walczak, F. A. Houle and J. B. Greenblatt, Energy Environ. Sci., 2014, 7, 3264–3278 RSC.
- R. Sathre, J. B. Greenblatt, K. Walczak, I. D. Sharp, J. C. Stevens, J. W. Ager and F. A. Houle, Energy Environ. Sci., 2016, 9, 803–819 RSC.
- O. Khaselev and J. A. Turner, Science, 1998, 280, 425–427 CrossRef CAS PubMed.
- M. Jouny, W. Luc and F. Jiao, Ind. Eng. Chem. Res., 2018, 57, 2165–2177 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Modelling of maximum achievable STH efficiencies, flow chart demonstrating how the LCH is calculated, mathematical calculations, Matlab files used for modelling. See DOI: 10.1039/d0se00524j |
| This journal is © The Royal Society of Chemistry 2020 |