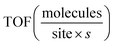Open Access Article
Open Access ArticleEffect of surface ligands on gold nanocatalysts for CO2 reduction†
Hongyu
Shang‡
 a,
Spencer K.
Wallentine‡
a,
Daniel M.
Hofmann
b,
Quansong
Zhu
a,
Catherine J.
Murphy
a,
Spencer K.
Wallentine‡
a,
Daniel M.
Hofmann
b,
Quansong
Zhu
a,
Catherine J.
Murphy
 b and
L. Robert
Baker
b and
L. Robert
Baker
 *a
*a
aThe Ohio State University, Columbus, Ohio, USA. E-mail: baker.2364@osu.edu
bUniversity of Illinois at Urbana-Champaign, Urbana, Illinois, USA
First published on 27th October 2020
Abstract
Nanoparticle catalysts display optimal mass activity due to their high surface to volume ratio and tunable size and structure. However, control of nanoparticle size requires the presence of surface ligands, which significantly influence catalytic performance. In this work, we investigate the effect of dodecanethiol on the activity, selectivity, and stability of Au nanoparticles for electrochemical carbon dioxide reduction (CO2R). Results show that dodecanethiol on Au nanoparticles significantly enhances selectivity and stability with minimal loss in activity by acting as a CO2-permeable membrane, which blocks the deposition of metal ions that are otherwise responsible for rapid deactivation. Although dodecanethiol occupies 90% or more of the electrochemical active surface area, it has a negligible effect on the partial current density to CO, indicating that it specifically does not block the active sites responsible for CO2R. Further, by preventing trace ion deposition, dodecanethiol stabilizes CO production on Au nanoparticles under conditions where CO2R selectivity on polycrystalline Au rapidly decays to zero. Comparison with other surface ligands and nanoparticles shows that this effect is specific to both the chemical identity and the surface structure of the dodecanethiol monolayer. To demonstrate the potential of this catalyst, CO2R was performed in electrolyte prepared from ambient river water, and dodecanethiol-capped Au nanoparticles produce more than 100 times higher CO yield compared to clean polycrystalline Au at identical potential and similar current.
1 Introduction
Nanoparticle catalysis benefits from inherently high surface to mass ratios and controllable surface structure,1–3 size,4,5 shape,3,6,7 and composition,4,8 which offer the ability to tune the activity and selectivity of heterogeneous catalytic systems.9 These properties are often controlled using small molecules or polymers, called capping agents, which coordinate to surface atoms during synthesis.10 Despite providing a high degree of synthetic control, from a catalysis perspective, organic capping agents are usually undesired because this inert layer occupies the majority of potentially active surface sites on the nanoparticle catalyst. Consequently, effort is usually made to remove the capping agent from a nanoparticle catalyst prior to use.10,11 However, there have been a number of studies which show that surface ligands can actually enhance selectivity12–17 and even activity.18–21 For example, capping agents have been shown to promote catalyst performance through selective site blocking,22,23 ligand substrate interactions,24,25 or by providing prolonged stability.21 These surface ligands can provide a homogeneous type handle to control heterogeneous catalysis.Many heterogeneous reactions, like carbon dioxide reduction (CO2R), have a strong surface structure dependence.26–28 Both theoretical and experimental studies have shown that CO2R on Au primarily occurs at undercoordinated sites (i.e. coordination number ≤ 7).29–33 Single crystal studies on Au show that these sites are at least 20-fold more active for CO2R than the more coordinated terrace sites.29 In that same study underpotential deposition also showed that these undercoordinated sites are the first to be poisoned by electrodeposition of divalent cations. It is well documented that electrodeposition of metal cations on an electrode surface during CO2R leads to catalyst deactivation,34–36 making electrolyte purity a difficult but important parameter to control. Considering the cost associated with water purification, this is an often overlooked challenge that is intimately related to the economic viability of aqueous CO2 conversion and utilization.37,38
In this work we investigate the influence of an organic passivation layer on the catalytic performance of small Au nanoparticles, having a 2 nm diameter, for electrochemical CO2R. We compare the activity, selectivity, and deactivation of the following systems: (1) as prepared Au nanoparticles containing a layer of 1-dodecanethiol (DDT-Au), (2) Au nanoparticles following removal of the dodecanethiol ligand by UV/ozone cleaning (UVO-Au), (3) Au nanoparticles of identical size prepared with a layer of triphenylphosphine in place of dodecanethiol (PPh3-Au), (4) CuAu bimetallic nanoparticles of identical size prepared with a layer of dodecanethiol (DDT-CuAu), and (5) polycrystalline Au (PC-Au) with and without a self-assembled monolayer (SAM) of dodecanethiol. Unless otherwise noted, measurements were performed in CO2-saturated 0.1 M sodium bicarbonate (pH = 6.8) at −1.1 V vs. RHE. To determine the effects of surface poisoning by electrodeposition of trace divalent metal ions, CO2R kinetics were compared in the presence and absence of 3.4 μM EDTA, which has been previously reported to prevent electroplating of divalent metal ions by strong chelation.39
We find that while dodecanethiol reductively desorbs from polycrystalline Au surfaces before the onset of CO2R, a stable population of dodecanethiol exists on the Au nanoparticle surface under CO2R reaction conditions at potentials as low as −1.1 V vs. RHE. Although the presence of dodecanethiol decreases the electrochemical active surface area of the Au nanoparticles, it has only a small effect on CO2R current. This is confirmed by similar initial partial currents of CO production on DDT-Au, PC-Au and UVO-Au. These findings suggest that while the dodecanethiol layer passivates the majority of the Au terrace sites, it specifically does not block the edge and/or corner sites, which are primarily responsible for CO2R. Additionally, not only does the capping layer not limit CO2R activity, we find that it greatly increases the catalyst stability by preventing the deposition of trace metal cations. In essence, this capping layer, which is permeable to CO2 is almost completely impermeable to divalent metal cations, providing enhanced stability with almost no decrease in catalytic activity. Finally, we show that this protecting effect is specific to both the chemical identity and the structure of the organic capping layer.
Specifically, we observe that on PPh3-Au faradaic selectivity to CO decays rapidly, indicating that, unlike DDT, PPh3 does not block ion deposition. Likewise, we find that DDT-capped CuAu nanoparticles also do not resist deactivation. On DDT-CuAu we observe reduced selectivity to CO and increased H2 evolution, consistent with the effects of Cu addition. However, focusing on catalyst stability, we find that the faradaic efficiency to CO on DDT-CuAu rapidly decays, similar to PC-Au and PPh3-Au. We attribute this to the reduced order and lower heat of adsorption of DDT on bimetallic CuAu nanoparticles compared to pure Au as characterized by vibrational sum frequency generation (SFG) spectroscopy.
2 Results and discussion
Nanoparticles were drop-casted onto a glassy carbon electrode prior to measuring CO2R kinetics in an electrochemical H-cell (see ESI Section 1 for additional Experimental details).† The nanoparticle mass loading was carefully selected to yield the same electrochemical active surface area (ECSA) for clean Au nanoparticles following ligand removal (i.e. UVO-Au) as for a clean PC-Au electrode. The capping agent was removed using UV generated ozone and washed with Milli-Q water.40 This process was sufficient to completely remove the dodecanethiol capping agent as confirmed by XPS measurements, which show complete elimination of the S 2p signal after cleaning (see Fig. S2 in ESI Section 1).† TEM images of UVO-Au immediately following DDT removal by UV–ozone shows that removal of the surface ligand results in nanoparticle aggregation, and we find that it is impossible to stabilize the nanoparticle morphology in the absence of the surface ligand (see Fig. S3†). This makes it difficult to quantify the exact fraction of the nanoparticle surface occupied by the DDT ligand because comparing ECSA before and after UV–ozone cleaning shows an increase in surface area resulting from ligand removal, but this is partially offset by a loss of geometric surface area caused by nanoparticle aggregation. Consequently, the ratio of ECSA measured before and after ligand removal represents a lower limit to the fraction of the Au nanoparticle occupied by the surface, and an even higher surface area would be observed after ligand removal if aggregation did not occur. Table 1 compares the ECSA measured by cyclic voltammetry, the partial current to CO measured by head space sampling, and the corresponding turnover frequency (TOF) using ECSA as an estimate for active site density. This comparison is provided for DDT-Au, PC-Au, and UVO-Au samples. As shown, the UVO-Au has a similar ECSA as the PC-Au, which is by design based on the mass loading of the drop cast samples. However, comparing the ECSA between DDT-Au and UVO-Au shows that the ECSA is 8-fold higher for UVO-Au than DDT-Au, indicating that the dodecanethiol capping agent occupies, as a minimum, at least 88% of the Au surface sites (for details of the ECSA measurements, see ESI Section 2).† This value represents the effective rather than absolute surface coverage and is defined by accessibility of redox species to the particle surface, compared to the absolute coverage, which is defined by the ratio between the number of Au surface atoms to the number of DDT molecules. Analysis of the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au atomic ratio measured by XPS allows us to additionally estimate the absolute surface coverage for DDT assuming a truncated polyhedron with a 1 nm radius as described in the ESI Section 3.† This analysis indicates that the absolute coverage of DDT on Au is ∼60%, and this value is in close agreement with previous reports by Hostetler et al.41 Comparison of the effective and absolute coverage indicates that closely spaced DDT molecules on a Au surface are sufficient to block access of solvated redox species to the electrode even if every Au site is not occupied and provides insight into mass transport through the cybotactic region as discussed further below.
Au atomic ratio measured by XPS allows us to additionally estimate the absolute surface coverage for DDT assuming a truncated polyhedron with a 1 nm radius as described in the ESI Section 3.† This analysis indicates that the absolute coverage of DDT on Au is ∼60%, and this value is in close agreement with previous reports by Hostetler et al.41 Comparison of the effective and absolute coverage indicates that closely spaced DDT molecules on a Au surface are sufficient to block access of solvated redox species to the electrode even if every Au site is not occupied and provides insight into mass transport through the cybotactic region as discussed further below.
Despite having only a small fraction of the total accessible ECSA, we find that the CO2R activity of DDT-Au is nearly identical to a clean PC-Au surface as measured by the partial current to CO. Normalizing the CO partial current to the ECSA, it is possible to estimate the TOF assuming an active site density on Au of approximately 1.2× 1015 sites per cm2 (see ESI Section 4).† This calculation provides a reasonable estimate of the average TOF of all exposed sites. The calculated TOF for DDT-Au is the highest (TOF = 14), where the exposed sites are found to be approximately 8-fold more active than the average activity of PC-Au (TOF = 1.8) and approximately 6-fold more active than the ensemble of sites present on UVO-Au (TOF = 2.4). These values represent initial kinetics during the first 20 min of reaction and are normalized to ECSA measurements of fresh DDT-Au, PC-Au, and UVO-Au. Below we consider the possibility that both reaction rate and ECSA may evolve in time during the reaction.
Particularly we note that thiols on Au have been reported to electrochemically desorb at reducing potentials. Consequently, we carefully consider how the dodecanethiol surface ligand coverage and associated ECSA is changing in time during CO2R reaction conditions. Using XPS, the ratio of S 2p and Au 4f XPS shows how the ligand coverage changes with time. Table S2 in Section 5 of the ESI† shows results for as-prepared DDT-Au nanoparticles and DDT-Au following 1 and 2 h reaction time. During the first 1 h of reaction time the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio decreases from 0.33 to 0.11 indicating that two thirds of dodecanethiol desorbs. However, after 2 h the S
Au ratio decreases from 0.33 to 0.11 indicating that two thirds of dodecanethiol desorbs. However, after 2 h the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio only drops slightly to 0.07, indicating that the remaining DDT is largely stable on the surface during extended reaction times. Post reaction TEM confirms that the size distribution of DDT-Au doesn't change after 2 h of reaction time (see Fig. S6 in the ESI Section 5†) providing further evidence that a sufficient fraction of dodecanethiol is stable on the nanoparticle surface during CO2R as required to stabilize the small nanoparticle size. In contrast, measurements performed on PC-Au functionalized with dodecanethiol (DDT-PC-Au), shows that on planar Au the dodecanethiol surface ligand is completely removed within 1 h of reaction time. This indicates that while dodecanethiol is not electrochemically stable at reducing potentials on planar Au, its stability is enhanced on small nanoparticles for at least 2 hours, after which we see a slow loss in activity, presumably due to gradual loss of the capping agent (see Fig. S8 ESI Section 5).†
Au ratio only drops slightly to 0.07, indicating that the remaining DDT is largely stable on the surface during extended reaction times. Post reaction TEM confirms that the size distribution of DDT-Au doesn't change after 2 h of reaction time (see Fig. S6 in the ESI Section 5†) providing further evidence that a sufficient fraction of dodecanethiol is stable on the nanoparticle surface during CO2R as required to stabilize the small nanoparticle size. In contrast, measurements performed on PC-Au functionalized with dodecanethiol (DDT-PC-Au), shows that on planar Au the dodecanethiol surface ligand is completely removed within 1 h of reaction time. This indicates that while dodecanethiol is not electrochemically stable at reducing potentials on planar Au, its stability is enhanced on small nanoparticles for at least 2 hours, after which we see a slow loss in activity, presumably due to gradual loss of the capping agent (see Fig. S8 ESI Section 5).†
To further understand this, we also measure cyclic voltammograms of DDT-Au and compare this to DDT-PC-Au. These data are presented in Fig. S7 in the ESI Section 5.† To summarize, on a planar dodecanethiol functionalized PC-Au electrode, we observe a redox wave associated with the reductive desorption and oxidative re-adsorption of dodecanethiol from the Au electrode. Although the reduction peak of this wave overlaps with H2 evolution and CO2 reduction, the oxidation peak associated with re-adsorption is clearly visible, and these findings are consistent with previous reports of CV cycling of SAMs on Au electrodes.42,43 In contrast, we find that the same redox feature is notably absent in the CV curve for DDT-Au, even though vibrational spectroscopy and XPS both confirm the presence of dodecanethiol on the nanoparticle surface. This indicates that the reductive desorption of dodecanethiol on DDT-Au nanoparticles is shifted to more negative potentials, and this is a result of the increased heat of adsorption of dodecanethiol on a small nanoparticle compared to bulk Au. For example, a study by Tsai et al.44 showed that for Au nanoparticles, capped with 11-mercaptoundecanoic acid, and ranging in size from 60 to 20 nm, the heat of adsorption of the thiol increases with decreasing nanoparticle size from 100 to 108 kJ mol−1. In this study we use much smaller nanoparticles of only 2 nm diameter, where the heat of adsorption is expected to be even stronger. While the actual value for heat of adsorption for dodecanethiol on ultrasmall nanoparticles is beyond the scope of the present paper, at this stage we confirm that a population of the dodecanethiol is stable on the DDT-Au nanoparticle surface under the CO2R reaction conditions studied here. The persistence of this capping agent is further verified by its ability to effectively block electrodeposition of metal ions on the DDT-Au nanoparticle surface as discussed below.
To investigate the effect of the dodecanethiol SAM on the electrodeposition of metal cations, we prepared an electrolyte solution of 0.1 M sodium bicarbonate to which we added 10 μM of ZnCl2. Concentrated Zn2+ was used because Zn2+ is a common contaminant in electrolyte solutions that is known to poison CO2R catalysts by electrodeposition during reaction, and because it is difficult to quantify the trace amount of ions in a deactivated sample by XPS.39 Using this intentionally contaminated electrolyte, the catalysts were exposed to CO2R reaction conditions at an applied potential of −1.1 V vs. RHE for 40 min, and then analyzed for the deposition of Zn using XPS. Results of the post-electrolysis XPS measurements are given in Fig. 1. Both the PC-Au and UVO-Au samples show large amounts of Zn, having a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au atomic ratio of 8.32 and 3.08, respectively, following electrolysis. By comparison, relatively little Zn is found to deposit on the DDT-Au catalyst following identical treatment, which has a Zn
Au atomic ratio of 8.32 and 3.08, respectively, following electrolysis. By comparison, relatively little Zn is found to deposit on the DDT-Au catalyst following identical treatment, which has a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio of only 0.51. We note that 10 μM ZnCl2 represents much higher than normal impurity concentration, but this serves to illustrate the effect of dodecanethiol to limit electroplating on Au during CO2R. Similar results are also observed for the electrodeposition of Cu2+ (see ESI Section 6).† These results show the ability of the dodecanethiol capping layer to effectively prevent the approach and deposition of solvated Zn2+ ions onto the Au surface. This effect is fortuitous given the observation that this same surface passivation layer does little to block the accessibility of CO2 to Au sites which are active for CO2R.
Au ratio of only 0.51. We note that 10 μM ZnCl2 represents much higher than normal impurity concentration, but this serves to illustrate the effect of dodecanethiol to limit electroplating on Au during CO2R. Similar results are also observed for the electrodeposition of Cu2+ (see ESI Section 6).† These results show the ability of the dodecanethiol capping layer to effectively prevent the approach and deposition of solvated Zn2+ ions onto the Au surface. This effect is fortuitous given the observation that this same surface passivation layer does little to block the accessibility of CO2 to Au sites which are active for CO2R.
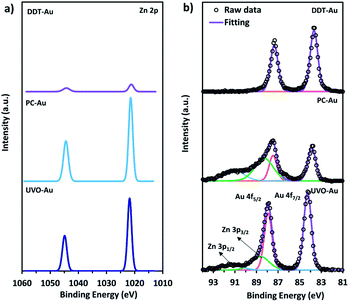 | ||
Fig. 1 XPS of (a) Zn 2p (b) Au 4f on DDT-Au, PC-Au and UVO-Au following electrolysis at −1.1 V vs. RHE in 0.1 M sodium bicarbonate electrolyte containing 10 μM of ZnCl2. Peak integration shows that the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au atomic ratio is 0.51, 8.32, and 3.08 on DDT-Au, PC-Au, and UVO-Au, respectively. The PC-Au and UVO-Au show additional peaks due to the overlap of the Au 4f5/2 peak with the Zn 3p edge.45 Au atomic ratio is 0.51, 8.32, and 3.08 on DDT-Au, PC-Au, and UVO-Au, respectively. The PC-Au and UVO-Au show additional peaks due to the overlap of the Au 4f5/2 peak with the Zn 3p edge.45 | ||
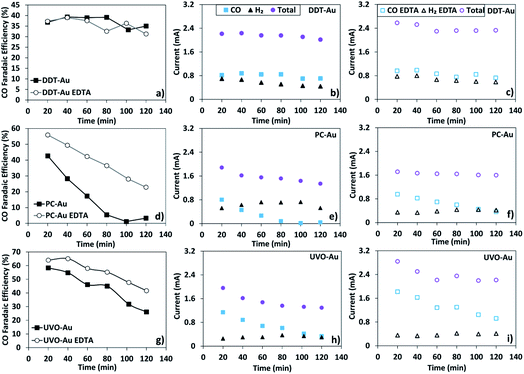
Given the ability of the capping layer to selectively block metal ion deposition during active CO2R, we investigated the kinetics of the same three catalysts described above in electrolyte containing only native ion contamination (i.e. no metal salts were intentionally introduced to the system). In these experiments, 18 MΩ water (Millipore) was used to prepare 0.1 M sodium bicarbonate electrolyte Sigma-Aldrich, (≥99.5%). Aside from using ultra-purified water and acid-cleaned glassware, no further steps were used to remove metal ion contamination. To illustrate the effects of surface poisoning by electrodeposition of trace divalent metal ions, CO2R kinetics were compared in the presence and absence of 3.4 μM EDTA, which has been previously reported to prevent electroplating of metal ions by strong chelation.39Fig. 2 shows the results of these experiments for DDT-Au (a–c), PC-Au (d–f), and UVO-Au (g–i). In these experiments, CO2R reaction kinetics are determined by head space sampling every 20 min during the course of a 2 h reaction. 1H-Nuclear Magnetic Resonance (NMR) analysis of the post-reaction electrolyte confirmed that no other products besides CO and H2 are produced, consistent with other reports of CO2R on Au.46–48
As shown, PC-Au initially produces CO with 45% faradaic selectivity. However, within approximately 100 min of reaction time, the faradaic selectivity to CO decreases to nearly zero and only the H2 evolution reaction remains active. This rapid deactivation is partially a result of metal ion contamination as evidenced by the comparison of this catalyst with and without the addition of 3.4 μM EDTA to the electrolyte. In the presence of EDTA, which strongly chelates any trace transition metal ions, we find that the CO2R activity decays much more slowly. The initial faradaic efficiency to CO during the first 20 min of reaction is also improved, presumably by preventing fast ion deposition during the initial several minutes of reaction, which occurs in the absence of EDTA.
In contrast, DDT-Au is completely resistant to deactivation by metal ion contamination. This can be seen by the constant faradaic selectivity during the course of the reaction as well as the nearly identical performance of this catalyst in the presence and absence of EDTA. Both these observations are consistent with the ability of dodecanethiol to block electrodeposition of metal ions at catalytic active sites on the Au surface as shown in Fig. 1 above.
Lastly, UVO-Au is initially the most selective catalyst for CO2R, having a faradaic selectivity of approximately 60%. This catalyst is also more active than PC-Au despite having a similar ECSA. Comparing the Au 4f XPS spectra of DDT-Au before and after ligand removal by UV–ozone treatment shows a shift in the spectrum consistent with oxidation of the Au nanoparticles (see Fig. S2 of the ESI Section 1).† It has been reported that oxide-derived Au is highly active for CO2 conversion to CO,49,50 and we expect this effect as well as ligand removal both contribute to the increase in activity upon cleaning. Although initially active, this catalyst is prone to deactivation and CO production drops from 60% to 30% faradaic selectivity during the course of the 2 h reaction. Although the stability of the UVO-Au catalyst is improved in the presence of EDTA, some deactivation is still observed. We attribute this deactivation, which occurs even in the presence of EDTA, to gradual reduction and time-dependent evolution of the surface structure for this oxide-derived Au catalyst during the course of the 2 h reaction.49
While the DDT-Au is more resistant to deactivation from ion deposition, it remains unclear whether this is a result of the ligand stabilized geometry, or a property of the ligand itself. This question is not trivial to answer because the ligand coverage and morphological stability are inherently coupled. To gain additional understanding about the influence of chemical identity and packing structure of the dodecanethiol capping agent on CO2R performance we prepared two additional samples: Au nanoparticles of similar size but with a triphenylphosphine rather than dodecanethiol surface layer (PPh3-Au) and CuAu bimetallic nanoparticles with a dodecanethiol surface layer (DDT-CuAu) also having the same 2 nm diameter. Although dodecanethiol forms a highly ordered SAM on a pure Au surface, addition of Cu atoms results in a significant decrease in the packing order. This can be observed in the vibrational SFG spectrum, where the relative intensity of the CH3 and CH2 stretches reflects the degree of packing order.51 Vibrational SFG spectra of a dodecanethiol SAM on PC-Au, DDT-Au, and DDT-CuAu are provided in the ESI Section 7.† These spectra confirm that while dodecanethiol forms a well-ordered monolayer on planar PC-Au, and only a small density of gauche defects are observed on DDT-Au, the degree of disorder increases significantly on CuAu bimetallic nanoparticles. This means that the comparison of DDT-Au with PPh3-Au allows us to observe the effect of chemical identity, and the comparison with DDT-CuAu allows us to observe the effect of dodecanethiol packing order on the resulting CO2R reaction kinetics.
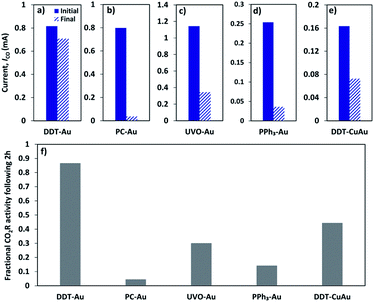
A summary of results for all catalysts tested here is provided in Fig. 3. Solid bars show the initial CO current measured during the first 20 min of reaction, and dash bars show the CO current following 2 h of reaction time. The difference between the solid and dash bars indicate the effects of catalyst deactivation. To illustrate this more clearly, Fig. 3f shows the relative CO2R activity following 2 h of reaction normalized to the initial rate, where a value of unity reflects no deactivation, and a value of zero reflects complete deactivation. We find that the PPh3-Au catalyst deactivates by approximately 85% during the experiment, indicating that triphenylphosphine is unable to protect the Au nanoparticle catalyst in the same manner as dodecanethiol. Similar to PPh3-Au, we find that DDT-CuAu also deactivates by approximately 55% over the course of the reaction.
As discussed above, it is not possible to perfectly disentangle the role of the DDT ligand and nanoparticle size in preventing ion deposition because ligand removal inherently alters the size distribution. However, both of these control experiments indicate that the ligand itself rather than the particle morphology is responsible for the observed resistance to deactivation. In both cases, particles of nearly identical size show significant deactivation suggesting that DDT ligand binding and structure, which is unique to DDT-Au compared to either PPh3-Au or DDT-CuAu protects the catalytic particle from trace metal ion deposition. Further support for this conclusion can be found in the literature, where it was shown by single crystal studies that underpotential ion deposition on Au electrodes occurs preferentially at undercoordinated sites.29,36 Because ultrasmall nanoparticles will have an increased density of low coordination sites as well as a higher surface energy, we believe it is unlikely that resistance to ion deposition is related to the small particle size and instead conclude that the surface ligand is primarily responsible for the observed resistance to deactivation.
As shown in Fig. 3e the total CO2R activity of the CuAu bimetallic catalyst is low compared to pure Au. This is because the addition of Cu results in decreased selectivity for CO and an increase in H2 evolution (see ESI Section 8).† These observations are consistent with previous reports, which show that very small Cu particles are also less selective for CO2R compared to larger Cu nanoparticles or bulk Cu catalysts.52 Unlike pure Au, DDT-CuAu also produces HCOO− at 2% faradaic efficiency (see ESI Section 9).† This is consistent with reports of CO2R on larger (8 nm) CuAu bimetallic particles, which also show small selectivity to HCOO−.48 However, focusing on the catalyst stability rather than overall activity, we find that the disordered structure of dodecanethiol on DDT-CuAu is less effective at preventing deactivation compared to DDT-Au. We attribute this to the combined effect of reduced order of DDT on bimetallic CuAu nanoparticles compared to pure Au as characterized by SFG spectroscopy as well as the weaker heat of adsorption of DDT on Cu compared to Au resulting in decreased catalyst stability. Of all the catalyst studied, only the DDT-Au sample resists significant deactivation, having approximately 90% of its initial activity following 2 h of reaction. These results indicate that both the chemical identity and packing order of the surface passivation layer contribute to the ability of dodecanethiol to protect the Au nanoparticles from deactivation.
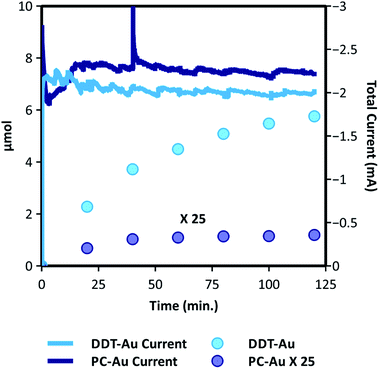
To demonstrate the ability of the dodecanethiol capping agent to prevent catalyst deactivation and preserve CO2R selectivity under non-ideal reaction conditions, we have performed kinetic measurements using electrolyte prepared with water obtained directly from the Olentangy River in Columbus, Ohio, USA. No purification except for particle filtration was performed. For reference, elemental analysis of this water is provided in the ESI Section 10.†53 This ambient water was used to prepare a 0.1 M sodium bicarbonate electrolyte, which was purged with CO2 prior to electrolysis at −1.1 V vs. RHE. Fig. 4 shows the results of CO2R performed using DDT-Au and PC-Au in this electrolyte. Above we showed that DDT-Au displays enhanced selectivity and stability compared to PC-Au even in electrolyte prepared using ultra-purified water, and a similar but much greater effect is observed in this native system. As shown in Fig. 4, the absolute current is nearly identical between these two catalysts, but CO2R activity to CO is more than 100 times greater on DDT-Au compared to PC-Au. Full kinetic plots showing the partial current to CO and H2 are provided in Fig. S12 of the ESI Section 10.† Not surprisingly, we observe that under these harsh conditions, DDT-Au shows signs of deactivation. However, we note that even following 2 h of electrolysis a steady production of CO is still observed, highlighting the usefulness of this system to operate under realistic conditions, not requiring tedious or expensive ultra-purification of reagents.
Au is one of the most selective catalysts for conversion of CO2 to CO. While Au is highly selective, it is also precious, which motivates improved efforts to reduce consumption and increase catalyst lifetime. Both these goals are effectively addressed through the use of ultrasmall DDT-Au nanoparticles as described here. First, the use of ultrasmall particles decreases Au consumption by maximizing catalyst dispersion compared to larger Au nanoparticles or polycrystalline films. Second, these ultrasmall particles are stabilized by the DDT surface ligand, which is earth-abundant and cheap compared to Au. Not only does DDT serve to stabilize the ultrasmall particle size, but as shown here, it effectively prevents deactivation of active Au surface sites by electrodeposition of trace metal ions. Accordingly, DDT-Au provides optimal use of Au as an electrocatalyst for CO2 conversion by simultaneously maximizing dispersion while also significantly enhancing catalyst lifetime.
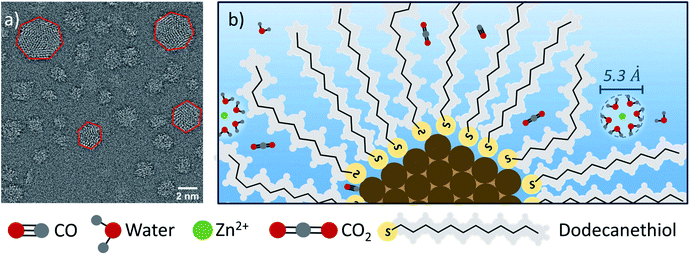
To consider the mechanism by which ligand-stabilized DDT-Au nanoparticles resist deactivation, we note that small nanoparticles have a high density of undercoordinated Au sites, which are known to be active for CO2R.29–33 While it is possible that these active sites are also inherently more resistant to deactivation, we believe that this is unlikely for two reasons: first, we observe that EDTA serves to slow the selectivity loss for PC-Au and UVO-Au suggesting that ion deposition is one of the primary contributors to deactivation. Second, ions preferentially deposit at undercoordinated sites during underpotential deposition,29 so one would expect that these active sites to be especially prone to poisoning by metal ions. To describe the selective nature of the dodecanethiol protecting layer, which is able to prevent deposition of metal ions, while allowing the approach of CO2 to Au active sites, we consider that the Au nanoparticles employed here can be described as multi-faceted polyhedrons as shown in the High-Angle Annular Dark-Field Scanning Transmission Electron Microscope (HAADF-STEM) of Fig. 5a. Additionally, we refer to the scale drawing in Fig. 5b. We note that dodecanethiol forms a highly ordered SAM on Au terraces,54 and we expect a large fraction of DDT to reside on the terraces. This is confirmed by vibrational SFG spectroscopy, which shows the presence of the SAM and indicates by the relative intensity of the CH2 and CH3 stretches, a high degree of order in the packing structure (see ESI Section 7).† However on small nanoparticles, surface ligands will minimize steric forces by striving to maintain a constant volume fraction in the cybotactic region. Although dodecanethiol will be unable to tightly pack across the edge between adjacent terraces, a constant volume fraction can be maintained by the formation of gauche defects. This is confirmed by comparing the SFG spectra of dodecanethiol on DDT-Au and PC-Au, which shows an increase in gauche defects on DDT-Au compared to PC-Au, and we expect these gauche defects are primarily located near the edge sites. This is depicted in the scaled schematic in Fig. 5b.
Based on the radius of a hydrated Zn2+ (or similar divalent transition metal ions), this solvation complex would be unable to approach either a terrace or an edge site given the density of the DDT monolayer. Although we have shown that the dodecanethiol coverage evolves during reaction, still we observe almost no catalyst deactivation for reaction times up to 2 h. These results suggest that there is a critical surface coverage that is necessary to effectively block deposition of divalent ions. This may be related to the ability of gauche defects to increase the volume fraction of dodecanethiol surface ligands, enabling ion blocking even as the dodecanethiol coverage decreases. Kinetic measurements indicate that during the first 2 h dodecanethiol stays above this critical surface coverage; however, this effect is lost at longer times (see Fig. S8 in the ESI Section 5).† From the S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio after 2 h of electrolysis, we can estimate this critical absolute coverage to be 13%.
Au ratio after 2 h of electrolysis, we can estimate this critical absolute coverage to be 13%.
It is not surprising that the effective dodecanethiol surface coverage from the perspective of a solvated ion would be larger than the absolute surface coverage. The hydrophobic nature of the dodecanethiol will repel the hydrated cations but would not necessarily block CO2, which is similarly hydrophobic.55,56 In fact, CO2 is about twice as soluble in dodecane as it is in water.56,57 This suggests that the dodecanethiol surface ligand would let CO2 diffuse to the nanoparticle, and may actually serve to concentrate CO2 near the catalyst surface. Consequently, Au edge sites, which are inaccessible to solvated transition metal ions are expected to be readily accessible to CO2. In this study, no effort has been made to optimize this effect by tuning the ligand composition or structure. For example, we expect a correlation between activity and chain length of a linear (or branched) thiol surface ligand based on the competition between mass transport of CO2 and metal ions, and we anticipate that such studies focused on optimization will become the focus of future work.
3 Conclusion
We have shown that, despite having a much smaller ECSA, Au nanoparticles passivated with a layer of dodecanethiol have similar activity for CO2 conversion to CO compared to clean polycrystalline Au as well as Au nanoparticles following ligand removal. Not only does the presence of the dodecanethiol surface ligand not limit CO partial current, we show that these ligand-capped Au nanoparticles are resistant to deactivation under conditions where the faradaic selectivity of polycrystalline Au rapidly decays to zero. We find that although dodecanethiol reductively desorbs from polycrystalline Au, on small Au nanoparticles a fraction of the ligand remains stable for >2 h reaction time at −1.1 V vs. RHE. The presence of this surface ligand is shown to significantly limit the deposition of metal ions on the Au nanoparticle catalyst during CO2R. These results indicate that dodecanethiol forms a selective membrane on Au nanoparticles, which prevents ion deposition while allowing nearly unhindered transport of CO2. Comparison with other surface ligands and nanoparticles shows that this protecting effect is specific to both the chemical identity and the surface structure of the dodecanethiol monolayer. When operating in an electrolyte obtained from an impure ambient water source, this DDT-Au catalyst produced a CO yield that is more than 100 times greater compared to PC-Au, illustrating that ligand passivation of a nanoparticle surface can greatly benefit catalytic activity despite the loss of ECSA. Given the cost associated with water ultra-purification, this has potential to help address the often overlooked challenge of electrolyte purity that is closely related to the economic viability of CO2 conversion.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Electrochemical catalysis and catalyst pre-and post-reaction characterization was supported by National Science Foundation under NSF award number 1665280. Nanoparticle synthesis was supported by National Science Foundation under NSF award number 1503408. XPS was performed at the Ohio State University Surface Analysis Laboratory. Film deposition was performed at the Ohio State University Nanosystems Laboratory. UVO-Au TEM and DDT-Au STEM imaging was performed at the OSU Center for Electron Microscopy and Analysis (CEMAS).Notes and references
- J. Yu, J. Low, W. Xiao, P. Zhou and M. Jaroniec, J. Am. Chem. Soc., 2014, 136, 8839–8842 CrossRef CAS.
- W. Luo, X. Nie, M. J. Janik and A. Asthagiri, ACS Catal., 2016, 6, 219–229 CrossRef CAS.
- J. Pal and T. Pal, Nanoscale, 2015, 7, 14159–14190 RSC.
- D. M. Hofmann, D. H. Fairbrother, R. J. Hamers and C. J. Murphy, ACS Appl. Nano Mater., 2019, 2, 3989–3998 CrossRef CAS.
- D. Gao, H. Zhou, J. Wang, S. Miao, F. Yang, G. Wang, J. Wang and X. Bao, J. Am. Chem. Soc., 2015, 137, 4288–4291 CrossRef CAS.
- S. Liu, H. Tao, L. Zeng, Q. Liu, Z. Xu, Q. Liu and J.-L. Luo, J. Am. Chem. Soc., 2017, 139, 2160–2163 CrossRef CAS.
- Z. Wu, S. Yang and W. Wu, Nanoscale, 2016, 8, 1237–1259 RSC.
- T. Witoon, N. Kachaban, W. Donphai, P. Kidkhunthod, K. Faungnawakij, M. Chareonpanich and J. Limtrakul, Energy Convers. Manage., 2016, 118, 21–31 CrossRef CAS.
- D. Kim, C. Xie, N. Becknell, Y. Yu, M. Karamad, K. Chan, E. J. Crumlin, J. K. Nørskov and P. Yang, J. Am. Chem. Soc., 2017, 139, 8329–8336 CrossRef.
- L. M. Rossi, J. L. Fiorio, M. A. Garcia and C. P. Ferraz, Dalton Trans., 2018, 47, 5889–5915 RSC.
- L. Altmann, S. Kunz and M. Baumer, J. Phys. Chem. C, 2014, 118, 8925–8932 CrossRef CAS.
- J. R. Pankhurst, Y. T. Guntern, M. Mensi and R. Buonsanti, Chem. Sci., 2019, 10, 10356–10365 RSC.
- C. Kim, T. Eom, M. S. Jee, H. Jung, H. Kim, B. K. Min and Y. J. Hwang, ACS Catal., 2017, 7, 779–785 CrossRef CAS.
- J. A. Trindell, J. Clausmeyer and R. M. Crooks, J. Am. Chem. Soc., 2017, 139, 16161–16167 CrossRef CAS.
- S. Jeong, G.-M. Kim, G.-S. Kang, C. Kim, H. Lee, W.-J. Kim, Y. K. Lee, S. Lee, H. Kim and H. K. Lim, et al. , J. Phys. Chem. C, 2019, 123, 29184–29191 CrossRef CAS.
- Z. Cai, Y. Zhang, Y. Zhao, Y. Wu, W. Xu, X. Wen, Y. Zhong, Y. Zhang, W. Liu and H. Wang, et al. , Nano Res., 2019, 12, 345–349 CrossRef CAS.
- H. Shang, T. Wang, J. Pei, Z. Jiang, D. Zhou, Y. Wang, H. Li, J. Dong, Z. Zhuang and W. Chen, et al. , Angew. Chem., Int. Ed., 2020 Search PubMed , https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202010903.
- Z. Wang, L. Wu, K. Sun, T. Chen, Z. Jiang, T. Cheng and W. A. Goddard III, J. Phys. Chem. Lett., 2018, 9, 3057–3061 CrossRef CAS.
- T. Taguchi, K. Isozaki and K. Miki, Adv. Mater., 2012, 24, 6462–6467 CrossRef CAS.
- I. Schrader, J. Warneke, J. Bakenkohler and S. Kunz, J. Am. Chem. Soc., 2015, 137, 905–912 CrossRef CAS.
- M. Moreno, F. J. Ibanez, J. B. Jasinski and F. P. Zamborini, J. Am. Chem. Soc., 2011, 133, 4389–4397 CrossRef CAS.
- S. Campisi, D. Ferri, A. Villa, W. Wang, D. Wang, O. Krocher and L. Prati, J. Phys. Chem. C, 2016, 120, 14027–14033 CrossRef CAS.
- P. Liu, R. Qin, G. Fu and N. Zheng, J. Am. Chem. Soc., 2017, 139, 2122–2131 CrossRef CAS.
- A. Villa, D. Wang, G. M. Veith, F. Vindigni and L. Prati, Catal. Sci. Technol., 2013, 3, 3036–3041 RSC.
- B. Wu, H. Huang, J. Yang, N. Zheng and G. Fu, Angew. Chem., Int. Ed., 2012, 51, 3440–3443 CrossRef CAS.
- R. K. Grasselli and A. Sleight, Structure-activity and selectivity relationships in heterogeneous catalysis, Elsevier, 1991 Search PubMed.
- T. Ressler, R. E. Jentoft, J. Wienold, F. Girgsdies, T. Neisius and O. Timpe, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 200, 165–170 CrossRef CAS.
- N. Todoroki, H. Tei, H. Tsurumaki, T. Miyakawa, T. Inoue and T. Wadayama, ACS Catal., 2019, 9, 1383–1388 CrossRef CAS.
- S. Mezzavilla, S. Horch, I. E. Stephens, B. Seger and I. Chorkendorff, Angew. Chem., 2019, 131, 3814–3818 CrossRef.
- W. Zhu, R. Michalsky, O. Metin, H. Lv, S. Guo, C. J. Wright, X. Sun, A. A. Peterson and S. Sun, J. Am. Chem. Soc., 2013, 135, 16833–16836 CrossRef CAS.
- W. Zhang, J. He, S. Liu, W. Niu, P. Liu, Y. Zhao, F. Pang, W. Xi, M. Chen and W. Zhang, et al. , Nanoscale, 2018, 10, 8372–8376 RSC.
- S. Back, M. S. Yeom and Y. Jung, ACS Catal., 2015, 5, 5089–5096 CrossRef CAS.
- W. Zhu, Y.-J. Zhang, H. Zhang, H. Lv, Q. Li, R. Michalsky, A. A. Peterson and S. Sun, J. Am. Chem. Soc., 2014, 136, 16132–16135 CrossRef CAS.
- Y. i. Hori, Modern aspects of electrochemistry, Springer, 2008, pp. 89–189 Search PubMed.
- Y. Hori, H. Konishi and T. Futamura, Electrochim. Acta, 2005, 50, 5354–5369 CrossRef CAS.
- J. Clavilier, J. Feliu and A. Aldaz, J. Electroanal. Chem. Interfacial Electrochem., 1988, 243, 419–433 CrossRef CAS.
- G. Klusewitz and J. Viegh, 13th Annual IEEE/SEMI Advanced Semiconductor Manufacturing Conference Advancing the Science and Technology of Semiconductor Manufacturing, ASMC, 2002, vol. 2002, pp. 340–346 Search PubMed.
- A. Bennett, Filtr. Sep., 2006, 43, 28–32 CrossRef.
- A. Wuttig and Y. Surendranath, ACS Catal., 2015, 5, 4479–4484 CrossRef CAS.
- C. G. Worley and R. W. Linton, J. Vac. Sci. Technol., A, 1995, 13, 2281–2284 CrossRef CAS.
- M. J. Hostetler, J. E. Wingate, C.-J. Zhong, J. E. Harris, R. W. Vachet, M. R. Clark, J. D. Londono, S. J. Green, J. J. Stokes and G. D. Wignall, et al. , Langmuir, 1998, 14, 17–30 CrossRef CAS.
- D.-F. Yang, C. Wilde and M. Morin, Langmuir, 1997, 13, 243–249 CrossRef CAS.
- M. M. Walczak, D. D. Popenoe, R. S. Deinhammer, B. D. Lamp, C. Chung and M. D. Porter, Langmuir, 1991, 7, 2687–2693 CrossRef CAS.
- D. Tsai, R. Zangmeister, L. Pease III, M. Tarlov and M. Zachariah, Langmuir, 2008, 24, 8483–8490 CrossRef CAS.
- Y. Cheng, S. Lu, W. Xu, H. Wen and J. Wang, J. Mater. Chem. A, 2015, 3, 16774–16784 RSC.
- S. Ringe, C. G. Morales-Guio, L. D. Chen, M. Fields, T. F. Jaramillo, C. Hahn and K. Chan, Nat. Commun., 2020, 11, 1–11 Search PubMed.
- B. A. Zhang, T. Ozel, J. S. Elias, C. Costentin and D. G. Nocera, ACS Cent. Sci., 2019, 5, 1097–1105 CrossRef CAS.
- D. Kim, J. Resasco, Y. Yu, A. M. Asiri and P. Yang, Nat. Commun., 2014, 5, 1–8 Search PubMed.
- Y. Chen, C. W. Li and M. W. Kanan, J. Am. Chem. Soc., 2012, 134, 19969–19972 CrossRef CAS.
- Z. Qi, J. Biener and M. Biener, ACS Appl. Energy Mater., 2019, 2, 7717–7721 CrossRef CAS.
- A. N. Bordenyuk, C. Weeraman, A. Yatawara, H. D. Jayathilake, I. Stiopkin, Y. Liu and A. V. Benderskii, J. Phys. Chem. C, 2007, 111, 8925–8933 CrossRef CAS.
- R. Reske, H. Mistry, F. Behafarid, B. Roldan Cuenya and P. Strasser, J. Am. Chem. Soc., 2014, 136, 6978–6986 CrossRef CAS.
- O. E. D. of Surface Water, Biological and water quality study of the Olentangy River and selected tributaries 1999: Delaware and Franklin Counties, Ohio EPA, Ohio, 2001 Search PubMed.
- N. Larsen, H. Biebuyck, E. Delamarche and B. Michel, J. Am. Chem. Soc., 1997, 119, 3017–3026 CrossRef CAS.
- D. Lide, CRC Handbook of Chemistry and Physics: Physical Constants of Inorganic Compounds, 2007, vol. 88, pp. 99–100 Search PubMed.
- P. G. Fogg, Carbon dioxide in non-aqueous solvents at pressures less than 200 kPa, Elsevier, 2017, vol. 50, pp. 19–27 Search PubMed.
- J. J. Carroll, J. D. Slupsky and A. E. Mather, J. Phys. Chem. Ref. Data, 1991, 20, 1201–1209 CrossRef CAS.
Footnotes |
| † The Electronic supplementary information (ESI) contains information regarding: (1) experimental details, (2) ECSA, (3) surface coverage calculation by XPS, (4) active site density calculation, (5) stability of DDT on Au nanoparticles, (6) XPS of Cu deposition, (7) DDT packing structure, (8) CO2R kinetics in the presence of EDTA, (9) identification of other products, (10) CO2R kinetics in ambient water. See DOI: 10.1039/d0sc05089j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |