Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-step synthesis of benzo[b]thiophenes by aryne reaction with alkynyl sulfides†
Tsubasa
Matsuzawa
,
Takamitsu
Hosoya
 and
Suguru
Yoshida
and
Suguru
Yoshida
 *
*
Laboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan. E-mail: s-yoshida.cb@tmd.ac.jp
First published on 1st September 2020
Abstract
An aryne reaction with alkynyl sulfides affording benzo[b]thiophenes is disclosed. A wide range of 3-substituted benzothiophenes were synthesized from easily available o-silylaryl triflates and alkynyl sulfides in a one-step intermolecular manner. The synthesis of diverse multisubstituted benzothiophene derivatives involving a pentacyclic compound was achieved by virtue of the good functional group tolerance and versatile C2 functionalizations.
Introduction
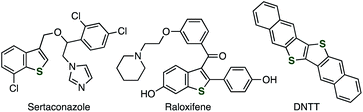
Benzo[b]thiophenes are a promising class of organosulfur compounds.1–3 In particular, multisubstituted benzothiophenes such as sertaconazole, raloxifene, and DNTT have served in a broad range of research fields including pharmaceutical sciences and materials chemistry (Fig. 1). In spite of their significance, although a number of benzothiophene syntheses such as transition-metal catalyzed reactions have been developed, the synthesis of multisubstituted benzothiophenes remains still difficult in terms of the applicable functional groups and substitution patterns due to the limited methods constructing the benzothiophene skeleton and introducing substituents.4,5 We herein present a novel approach to form benzothiophene scaffold from easily available alkynyl sulfides and aryne precursors.Reactions of aryne intermediates with a variety of sulfides are attractive methods for preparing a wide range of organosulfur compounds (Fig. 2).6–9 In the 1980s, a pioneering study on the reaction between sulfides and benzyne intermediate (I) generated from benzenediazonium-2-carboxylate was reported by Nakayama and coworkers (Fig. 2A).9a,9b Recently, an elegant difunctionalization of aryne intermediates was achieved by Studer and coworkers, in which C–S and C–C formations and C–S cleavage simultaneously took place (Fig. 2B).9h Benzothiophene synthesis from o-silylaryl triflates and acyl-substituted ketene dithioacetals was developed by Singh and coworkers in 2016 through the formation of benzothiophene skeleton via aryne intermediates, and further addition with aryne intermediates (Fig. 2C).9o On the basis of our recent studies of synthetic aryne chemistry,10 we envisioned that benzothiophenes can be synthesized from aryne precursors and alkynyl sulfides,11,12 starting from the nucleophilic attack of the sulfur or carbon of alkynyl sulfides to electrophilic aryne intermediates followed by ring-closure (Fig. 2D).
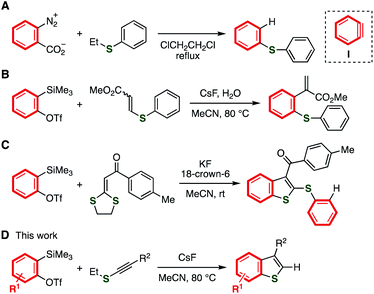 | ||
| Fig. 2 Aryne reactions with organosulfur compounds. (A) Nakayama's study. (B) Studer's work. (C) Singh's work. (D) This work. | ||
Results and discussion
First, a reaction between 2-chloro-6-(trimethylsilyl)phenyl triflate (1a) and ethyl p-tolylethynyl sulfide (2a) was examined (Fig. 3A and B). As a result, we found that treatment of a mixture between aryne precursor 1a and alkynyl sulfide 2a with cesium fluoride in hot acetonitrile provided 3-(4-tolyl)-4-chlorobenzo[b]thiophene (3a) in high yield. The construction of benzothiophene scaffold was accomplished by C–S bond formation selectively at C1 of 3-chlorobenzyne (II), C–C bond formation, protonation, and deethylation, where the regioisomer was not detected. When the reaction was conducted on a larger scale using 2 mmol of alkynyl sulfide 2a, the yield of benzothiophene 3a was slightly decreased. Increasing concentration from 0.05 M to 0.2 or 0.5 M slightly reduced the yield of 3a (64% or 52%, respectively).13 Benzothiophene 3a was also obtained in moderate to good yields even when the amount of aryne precursor 1a was decreased from 3.0 equiv. to 2.0, 1.5, and 1.2 equiv.13 These results show good practicality of the benzothiophene synthesis from o-silylaryl triflates 1 and alkynyl sulfides 2. The reaction of aryne precursor 1a with methyl, isopropyl, or benzyl p-tolylethynyl sulfides instead of 2a also afforded benzothiophene 3a, although 3a was not detected in the case of p-tolyl p-tolylethynyl sulfide.13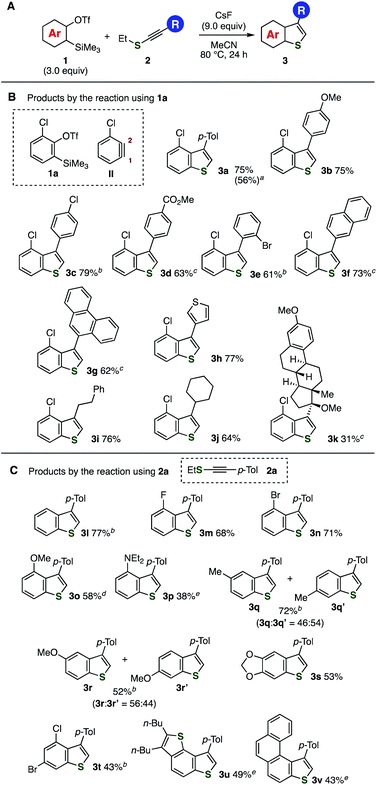 | ||
| Fig. 3 Synthesis of benzothiophenes 3 from o-silylaryl triflates 1 and alkynyl sulfides 2. See, the ESI† for the structures of 1 and 2. (A) General scheme. (B) Reactions of various alkynyl sulfides 2 with 1a. (C) Reactions of various o-silylaryl triflates 1 with 2a. a Isolated yield using 2.0 mmol of 2a in parentheses. b The reactions were performed in 1,4-dioxane at 110 °C. c The reactions were performed using 5.0 equiv. of 1 and 15 equiv. of CsF. d The reaction was performed at rt. e The reactions were performed using 5.0 equiv. of 1 and 15 equiv. of CsF in 1,4-dioxane at 110 °C. | ||
A broad range of 3-aryl- and 3-alkyl-substituted benzo[b]thiophenes were prepared from aryne precursor 1a and various alkynyl sulfides 2 (Fig. 3A and B). For example, electron-donating methoxy- and electron-withdrawing chloro- and methoxycarbonyl-substituted arylethynyl ethyl sulfides smoothly reacted with aryne intermediates to afford benzo[b]thiophenes 3b–d without damaging these functional groups. Bulky 2-bromophenylethynyl ethyl sulfide also participated in the reaction providing 3e in good yield. Furthermore, benzothiophenes 3f and 3g having π-extended aromatics and 3h possessing heteroaromatic thiophene ring were synthesized efficiently from the corresponding alkynyl sulfides. The reaction of primary and secondary alkylethynyl ethyl sulfides with aryne intermediates also proceeded under the same conditions to afford 3i and 3j in high yields. Moreover, we succeeded in the synthesis of benzothiophene 3k from alkynyl sulfide 2k prepared from an ethynylestradiol derivative. Since a wide variety of alkynyl sulfides were easily available from the corresponding terminal alkynes and thiosulfonates catalyzed by copper as we recently reported,12e this method enables the synthesis of diverse 3-substituted benzothiophenes.
Diverse aryne precursors were applicable to the one-step benzothiophene synthesis enabling to prepare a variety of benzothiophenes 3l–v (Fig. 3A and C). Not only simple benzyne but also 3-fluoro-, 3-bromo, 3-methoxy, and 3-aminobenzyne intermediates reacted with alkynyl sulfides 2a to furnish 3l–p in moderate to good yields leaving these functional groups untouched, in which regioisomers were not detected. Especially, selective C–S bond formation proceeded in the reaction of aryne intermediates bearing functional groups at 3-position, showing that the benzothiophene formation triggered by the nucleophilic attack of the sulfur atom onto C1 of the 3-substituted aryne intermediates due to the inductive effect of fluorine, bromine, oxygen, and nitrogen.14 Reactions of 4-methoxy- and 4-methylbenzyne with alkynyl sulfide 2a furnished ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of regioisomers of benzothiophenes 3q and 3r in moderate to high yields. Trisubstituted benzothiophenes 3s and 3t were also synthesized from the corresponding o-silylaryl triflates. It is worthy to note that the synthesis of π-conjugated benzothiophenes 3u and 3v was accomplished from the corresponding benzothiophene- and phenanthrene-type o-silylaryl triflates.10c The broad scope of the synthesizable benzothiophenes clearly demonstrated a benefit of this method by virtue of the recent remarkable advancement of the accessibility of o-silylaryl triflates and predictable reactivity of aryne intermediates by the aryne distortion model.14
1 mixtures of regioisomers of benzothiophenes 3q and 3r in moderate to high yields. Trisubstituted benzothiophenes 3s and 3t were also synthesized from the corresponding o-silylaryl triflates. It is worthy to note that the synthesis of π-conjugated benzothiophenes 3u and 3v was accomplished from the corresponding benzothiophene- and phenanthrene-type o-silylaryl triflates.10c The broad scope of the synthesizable benzothiophenes clearly demonstrated a benefit of this method by virtue of the recent remarkable advancement of the accessibility of o-silylaryl triflates and predictable reactivity of aryne intermediates by the aryne distortion model.14
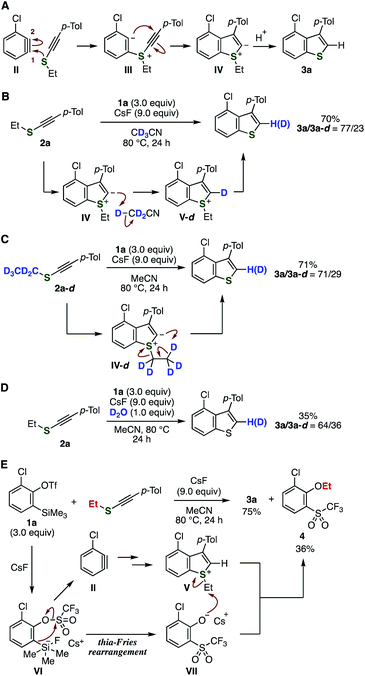
A plausible reaction mechanism is shown in Fig. 4A. First, the nucleophilic addition of the sulfur atom of alkynyl sulfides onto arynes and following cyclization to the alkyne carbon construct the benzothiophene skeleton. Then, protonation of the resulting zwitterionic intermediate IV leads to benzothiophene 3a. To examine the proton source, we then performed control experiments using deuterated compounds (Fig. 4B–D). Treatment of o-silylaryl triflate 1a and alkynyl sulfide 2a dissolved in CD3CN with cesium fluoride provided benzothiophene 3a with partial incorporation of deuterium through sulfonium intermediate V-d (Fig. 4B).9b,15 The reaction using deuterium-labeled ethyl p-tolylethynyl sulfide 2a-d in acetonitrile also resulted in partial deuterium incorporation, suggesting intramolecular deuteration of zwitterionic intermediate IV-d with liberating ethylene (Fig. 4C).9m An alternative proton source would be water in the reagents used, since deuterium was incorporated when the reaction was performed in the presence of deuterium oxide (Fig. 4D). Moreover, 2-chloro-6-(trifluoromethanesulfonyl)phenyl ethyl ether (4) was detected as a side-product in the synthesis of 3a, clearly showing that 2-chloro-6-(trifluoromethanesulfonyl)phenolate intermediate VII was generated from 1avia the thia-Fries rearrangement of silicate intermediate VI,16 and cesium phenolate VII was involved in the deethylation process of sulfonium intermediate V (Fig. 4E). Thus, possible protonation and deethylation mechanisms from zwitterionic intermediate IV were supported by these results.
Various C2-functionalizations of benzothiophene 3a allowed for the preparation of a wide range of 2,3,4-trisubstituted benzothiophenes (Fig. 5).17 For example, selective deprotonation of 3a with lithium diisopropylamide (LDA) proceeded efficiently (Fig. 5A). Sulfanylation, iodination, and ethoxycarbonylation of the resulting 2-benzothiophenyllithium VIII provided benzothiophenes 5a–c in good yields. Furthermore, S-oxidation followed by the Pummerer-type C2-arylation with phenol through [3,3]-sigmatropic rearrangement selectively afforded benzothiophene 7 (Fig. 5B).17h Additionally, treatment of o-silylaryl triflate 1a and alkynyl sulfide 2a with cesium fluoride under carbon dioxide furnished benzothiophene 5c having an ester moiety albeit in low yield, where C–C bond formation of zwitterionic intermediate IV and subsequent migration of the ethyl group occurred (Fig. 5C).
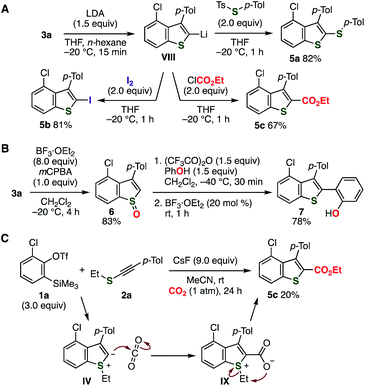 | ||
| Fig. 5 C2-functionalizations of benzothiophene 3a. (A) Transformations through C2-deprotonation. (B) Arylation via Pummerer-type activation. (C) Direct ester formation. | ||
We succeeded in the preparation of C3-functionalized benzothiophene 10 from alkyne 8 and aryne precursor 1a through C–C cleavage of carboxylic acid 9 by virtue of the good accessibility of alkynyl sulfides and broad substrate scope of the aryne reaction (Fig. 6A). Indeed, latently transformable alkynyl sulfide 2l was synthesized from terminal alkyne 8 and S-ethyl p-toluenethiosulfonate catalyzed by CuI/xantphos under mild conditions.12e Following aryne reaction between 2l and o-silylaryl triflate 1a and subsequent removal of the tetrahydropyranyl (THP) group successfully afforded benzothiophene 3w having chloro and hydroxymethyl groups. Then, carboxylic acid 9 was prepared by oxidation with tert-butyl hydroperoxide catalyzed by copper(II) bromide.18 Considering that recent remarkable achievements for transformations of the carboxy group into a range of functional groups such as halogens, phosphorus moieties, and aryl groups through C–C cleavage,19 diverse C3-functionalized benzothiophenes will be synthesized from benzothiophene 9. For example, decarboxylative iodination of 9 took place smoothly to provide 4-chloro-3-iodobenzo[b]thiophene (10) in high yield.19b Thus, a wide variety of benzothiophenes can be synthesized through the aryne reaction between alkynyl sulfide 2l and o-silylaryl triflates and decarboxylative transformations.
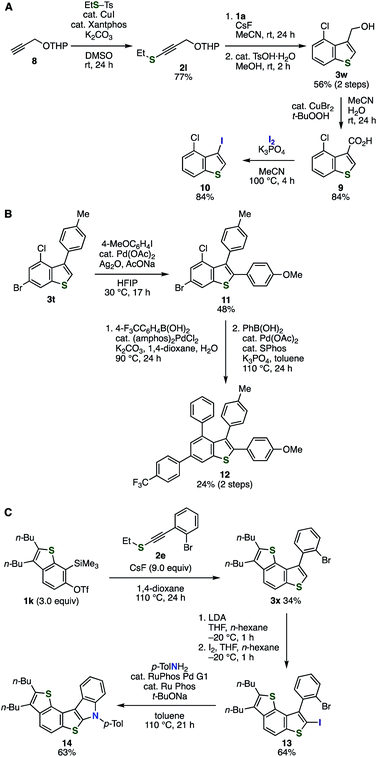 | ||
| Fig. 6 Applications of the benzothiophene synthesis. (A) Benzothiophene synthesis through C3-functionalizations. (B) Synthesis of tetraarylbenzothiophene 12. (C) Synthesis of pentacyclic compound 14. | ||
We achieved the synthesis of benzothiophene 12 bearing four different aryl groups by virtue of the halogen-tolerated benzothiophene synthesis and the versatility of C2-position (Fig. 6B). Indeed, direct C–H arylation of benzothiophene 3t with 4-iodoanisole proceeded smoothly to provide 11 in moderate yield keeping bromo and chloro groups intact.17i Then, a sequential Suzuki–Miyaura cross-coupling of 11 with 4-(trifluoromethyl)phenyboronic acid and phenylboronic acid at the bromo and chloro group, respectively, successfully furnished 2,3,4,6-tetraarylbenzothiophene 12. This modular synthetic route would enable the preparation of diverse multi-arylated benzothiophenes using various alkynyl sulfides, aryl iodides, and arylboronic acids.20
The good accessibility of o-silylaryl triflates and alkynyl sulfides realized the synthesis of polycyclic aromatic compound 14 (Fig. 6C). Firstly, the treatment of 6,7-thienobenzyne precursor 1k and alkynyl sulfide 2e with cesium fluoride afforded dithienobenzene 3x in moderate yield. Then, C2-iodination was realized by deprotonation with LDA followed by the addition of iodine. Finally, palladium-catalyzed amination at C2 of benzothiophene with p-toluidine and subsequent cyclization proceeded efficiently to afford pentahelicene analog 14. This result clearly demonstrated an advantage of the benzothiophene synthesis by the aryne reaction with alkynyl sulfides enabling to prepare π-extended benzothiophenes having functional groups such as halogens. The benzothiophene synthesis will serve in the synthesis of various polyaromatic analogs containing benzothiophene skeleton.21
Conclusions
In summary, we have developed a facile one-step synthetic method of benzothiophenes from o-silylaryl triflates and alkynyl sulfides. The wide scope of the benzothiophene synthesis and the versatile C2-functionalizations enabled the synthesis of a variety of multisubstituted benzothiophenes, which is difficult by the conventional methods. Further studies to clarify the reaction mechanism and to expand synthesizable multisubstituted benzothiophenes involving three-component couplings, and applications to synthesize analogs of bioactive compounds are currently underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Yuki Sakata at Tokyo Medical and Dental University for HRMS analyses. This work was supported by JSPS KAKENHI Grant Numbers JP19K05451 (C; S. Y.), JP18H02104 (B; T. H.), JP18H04386 (Middle Molecular Strategy; T. H.), and 19J14128 (JSPS Research Fellow; T. M.); the Naito Foundation (S. Y.); the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP20am0101098 (Platform Project for Supporting Drug Discovery and Life Science Research, BINDS) and JP20gm0910007 (CREST); and the Cooperative Research Project of Research Center for Biomedical Engineering.Notes and references
- (a) J. D. Croxtall and G. L. Plosker, Drugs, 2009, 69, 339 CrossRef CAS PubMed; (b) S. Dadiboyena, Eur. J. Med. Chem., 2012, 51, 17 CrossRef CAS PubMed; (c) R. Romagnoli, P. G. Baraldi, C. Lopez-Cara, D. Preti, M. A. Tabrizi, J. Balzarini, M. Bassetto, A. Brancale, X.-H. Fu, Y. Gao, J. Li, S.-Z. Zhang, E. Hamel, R. Bortolozzi, G. Basso and G. Viola, J. Med. Chem., 2013, 56, 9296 CrossRef CAS PubMed; (d) R. S. Keri, K. Chand, S. Budagumpi, S. B. Somappa, S. A. Patil and B. M. Nagaraja, Eur. J. Med. Chem., 2017, 138, 1002 CrossRef CAS PubMed; (e) Y. Lu, L. M. Gutgesell, R. Xiong, J. Zhao, Y. Li, C. I. Rosales, M. Hollas, Z. Shen, J. Gordon-Blake, K. Dye, Y. Wang, S. Lee, H. Chen, D. He, O. Dubrovyskyii, H. Zhao, F. Huang, A. W. Lasek, D. A. Tonetti and G. R. J. Thatcher, J. Med. Chem., 2019, 62, 11301 CrossRef CAS PubMed.
- For our study on bioactive benzo[b]thiophenes, see: S. Shimizu, T. Hosoya, M. Murohashi and S. Yoshida, WO2013/118842, 2013.
- (a) T. Yamamoto and K. Takimiya, J. Am. Chem. Soc., 2007, 129, 2224 CrossRef CAS PubMed; (b) H. Takenaka, T. Ogaki, C. Wang, K. Kawabata and K. Takimiya, Chem. Mater., 2019, 31, 6696 CrossRef CAS.
- For a review on benzothiophene synthesis, see: B. Wu and N. Yoshikai, Org. Biomol. Chem., 2016, 14, 5402 RSC.
- For examples of benzothiophene synthesis, see: (a) R. C. Larock and D. Yue, Tetrahedron Lett., 2001, 42, 6011 CrossRef CAS; (b) S. Yoshida, H. Yorimitsu and K. Oshima, Org. Lett., 2007, 9, 5573 CrossRef CAS PubMed; (c) C. S. Bryan, J. A. Braunger and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 7064 CrossRef CAS PubMed; (d) L.-L. Sun, C.-L. Deng, R.-Y. Tang and X.-G. Zhang, J. Org. Chem., 2011, 76, 7546 CrossRef CAS PubMed; (e) T. Kunz and P. Knochel, Angew. Chem., Int. Ed., 2012, 51, 1958 CrossRef CAS PubMed; (f) D. P. Hari, T. Hering and B. König, Org. Lett., 2012, 14, 5334 CrossRef CAS PubMed; (g) K. Liu, F. Jia, H. Xi, Y. Li, X. Zheng, Q. Guo, B. Shen and Z. Li, Org. Lett., 2013, 15, 2026 CrossRef CAS PubMed; (h) B. Wu and N. Yoshikai, Angew. Chem., Int. Ed., 2013, 52, 10496 CrossRef CAS PubMed; (i) A. Acharya, S. V. Kumar and H. Ila, Chem.–Eur. J., 2015, 21, 17116 CrossRef CAS PubMed; (j) Y. Masuya, M. Tobisu and N. Chatani, Org. Lett., 2016, 18, 4312 CrossRef CAS PubMed; (k) A. J. Eberhart, H. Shrives, Y. Zhang, A. Carrër, A. V. S. Parry, D. J. Tate, M. L. Turner and D. J. Procter, Chem. Sci., 2016, 7, 1281 RSC; (l) L. Gao, B. Chang, W. Qiu, L. Wang, X. Fu and R. Yuan, Adv. Synth. Catal., 2016, 358, 1202 CrossRef CAS; (m) L. Meng, T. Fujikawa, M. Kuwayama, Y. Segawa and K. Itami, J. Am. Chem. Soc., 2016, 138, 10351 CrossRef CAS PubMed; (n) S. Yugandar, S. Konda and H. Ila, Org. Lett., 2017, 19, 1512 CrossRef CAS PubMed; (o) Z. Zheng, L. Chen, C. Qian, X. Zhu, Y. Yang, J. Liu, Y. Yang and Y. Liang, Org. Biomol. Chem., 2018, 16, 8020 RSC; (p) S. Mikami, H. Tanaka, H. Kishi, S. Yoshida and K. Toyota, Heterocycles, 2018, 96, 1529 CrossRef CAS; (q) R. Zhu, Z. Liu, J. Chen, X. Xiong, Y. Wang, L. Huang, J. Bai, Y. Dang and J. Huang, Org. Lett., 2018, 20, 3161 CrossRef CAS PubMed; (r) J. Yan, A. P. Pulis, G. J. P. Perry and D. J. Procter, Angew. Chem., Int. Ed., 2019, 58, 15675 CrossRef CAS PubMed; (s) A. Mizukami, M. Tsugita, M. Shimora, S. Tanaka, N. Hayama, T. Kimachi and K. Inamoto, Chem. Lett., 2019, 48, 468 CrossRef CAS; (t) S. Uchida, H. Kinoshita and K. Miura, Org. Lett., 2020, 22, 3123 CrossRef CAS PubMed; (u) S. Moon, M. Kato, Y. Nishii and M. Miura, Adv. Synth. Catal., 2020, 362, 1669 CrossRef CAS.
- For selected recent reviews on arynes, see: (a) P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550 CrossRef CAS PubMed; (b) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Org. Biomol. Chem., 2013, 11, 191 RSC; (c) S. Yoshida and T. Hosoya, Chem. Lett., 2015, 44, 1450 CrossRef CAS; (d) J.-A. García-López and M. F. Greaney, Chem. Soc. Rev., 2016, 45, 6766 RSC; (e) J. Shi, Y. Li and Y. Li, Chem. Soc. Rev., 2017, 46, 1707 RSC; (f) F. I. M. Idiris and C. R. Jones, Org. Biomol. Chem., 2017, 15, 9044 RSC; (g) T. Roy and A. T. Biju, Chem. Commun., 2018, 54, 2580 RSC; (h) S. Yoshida, Bull. Chem. Soc. Jpn., 2018, 91, 1293 CrossRef CAS; (i) H. Takikawa, A. Nishii, T. Sakai and K. Suzuki, Chem. Soc. Rev., 2018, 47, 8030 RSC; (j) Y. Nakamura, S. Yoshida and T. Hosoya, Heterocycles, 2019, 98, 1623 CrossRef.
- For selected aryne chemistries, see: (a) U. N. Rao and E. Biehl, J. Org. Chem., 2002, 67, 3409 CrossRef CAS PubMed; (b) Y. Chen and M. C. Willis, Org. Lett., 2015, 17, 4786 CrossRef CAS PubMed; (c) J. Shi, D. Qiu, J. Wang, H. Xu and Y. Li, J. Am. Chem. Soc., 2015, 137, 5670 CrossRef CAS PubMed; (d) W. Ding, A. Yu, L. Zhang and X. Meng, Org. Lett., 2019, 21, 9014 CrossRef CAS PubMed; (e) M. Mesgar, J. Nguyen-Le and O. Daugulis, J. Am. Chem. Soc., 2018, 140, 13703 CrossRef CAS PubMed; (f) R. N. Gaykar, A. Guin, S. Bhattacharjee and A. T. Biju, Org. Lett., 2019, 21, 9613 CrossRef CAS PubMed; (g) J. He, Z. Jia, H. Tan, X. Luo, D. Qiu, J. Shi, H. Xu and Y. Li, Angew. Chem., Int. Ed., 2019, 58, 18513 CrossRef CAS PubMed; (h) H. Takikawa, A. Nishii and K. Suzuki, Chem. Sci., 2019, 10, 3840 RSC; (i) E. Yen-Pon, P. A. Champagne, L. Plougastel, S. Gabillet, P. Thuéry, M. Johnson, G. Muller, G. Pieters, F. Taran, K. N. Houk and D. Audisio, J. Am. Chem. Soc., 2019, 141, 1435 CrossRef CAS PubMed; (j) X. Xiao and T. R. Hoye, J. Am. Chem. Soc., 2019, 141, 9813 CrossRef CAS PubMed; (k) S. K. Thompson and T. R. Hoye, J. Am. Chem. Soc., 2019, 141, 19575 CrossRef CAS PubMed; (l) T. Hosokawa, T. Asada and K. Kamikawa, J. Phys. Chem. A, 2020, 124, 652 CrossRef CAS PubMed; (m) T. Ikawa, S. Masuda and S. Akai, Chem.–Eur. J., 2020, 26, 4320 CrossRef CAS PubMed; (n) W. Cao, S.-L. Niu, L. Shuai and Q. Xiao, Chem. Commun., 2020, 56, 972 RSC; (o) H. Hazarika and P. Gogoi, Org. Biomol. Chem., 2020, 18, 2727 RSC; (p) S. Ghorai, Y. Lin, Y. Xia, D. J. Wink and D. Lee, Org. Lett., 2020, 22, 626 CrossRef CAS PubMed; (q) S. Cho and Q. Wang, Org. Lett., 2020, 22, 1670 CrossRef CAS PubMed; (r) H. Fujimoto, M. Kusano, T. Kodama and M. Tobisu, Org. Lett., 2020, 22, 2293 CrossRef CAS PubMed; (s) H. Takikawa, A. Nishii, H. Takiguchi, H. Yagishita, M. Tanaka, K. Hirano, M. Uchiyama, K. Ohmori and K. Suzuki, Angew. Chem., Int. Ed., 2020, 59, 12440 Search PubMed.
- For reviews on reactions between arynes and organosulfur compounds., see: (a) J. Nakayama and K. Akimoto, Sulfur Rep., 1994, 16, 61 CrossRef CAS; (b) T. Matsuzawa, S. Yoshida and T. Hosoya, Tetrahedron Lett., 2018, 59, 4197 CrossRef CAS.
- (a) J. Nakayama, T. Fujita and M. Hoshino, Chem. Lett., 1982, 11, 1777 CrossRef; (b) J. Nakayama, T. Fujita and M. Hoshino, Chem. Lett., 1983, 12, 249 CrossRef; (c) S. Yoshida, K. Uchida, K. Igawa, K. Tomooka and T. Hosoya, Chem. Commun., 2014, 50, 15059 RSC; (d) H.-D. Xu, M.-Q. Cai, W.-J. He, W.-H. Hu and M.-H. Shen, RSC Adv., 2014, 4, 7623 RSC; (e) M. Pawliczek, L. K. B. Garve and D. B. Werz, Chem. Commun., 2015, 51, 9165 RSC; (f) J. Chen, V. Palani and T. R. Hoye, J. Am. Chem. Soc., 2016, 138, 4318 CrossRef CAS PubMed; (g) L.-R. Wen, N.-N. Man, W.-K. Yuan and M. Li, J. Org. Chem., 2016, 81, 5942 CrossRef CAS PubMed; (h) Y. Li, C. Mück-Lichtenfeld and A. Studer, Angew. Chem., Int. Ed., 2016, 55, 14435 CrossRef CAS PubMed; (i) M. Thangaraj, R. N. Gaykar, T. Roy and A. T. Biju, J. Org. Chem., 2017, 82, 4470 CrossRef CAS PubMed; (j) M. M. Ahire, R. Khan and S. B. Mhaske, Org. Lett., 2017, 19, 2134 CrossRef CAS PubMed; (k) X.-B. Xu, Z.-H. Lin, Y. Liu, J. Guo and Y. He, Org. Biomol. Chem., 2017, 15, 2716 RSC; (l) J. Tan, T. Zheng, K. Xu and C. Liu, Org. Biomol. Chem., 2017, 15, 4946 RSC; (m) L. Zhang, X. Li, Y. Sun, W. Zhao, F. Luo, X. Huang, L. Lin, Y. Yang and B. Peng, Org. Biomol. Chem., 2017, 15, 7181 RSC; (n) H. Jian, Q. Wang, W.-H. Wang, Z.-J. Li, C.-Z. Gu, B. Dai and L. He, Tetrahedron, 2018, 74, 2876 CrossRef CAS; (o) P. Garg and A. Singh, Org. Lett., 2018, 20, 1320 CrossRef CAS PubMed; (p) T. Zheng, J. Tan, R. Fan, S. Su, B. Liu, C. Tan and K. Xu, Chem. Commun., 2018, 54, 1303 RSC; (q) R. Fan, B. Liu, T. Zheng, K. Xu, C. Tan, T. Zeng, S. Su and J. Tan, Chem. Commun., 2018, 54, 7081 RSC; (r) Y. Nakamura, Y. Miyata, K. Uchida, S. Yoshida and T. Hosoya, Org. Lett., 2019, 21, 5252 CrossRef CAS PubMed; (s) P. Singh, A. G. Cairns, D. E. Adolfsson, J. Ådén, U. H. Sauer and F. Almqvist, Org. Lett., 2019, 21, 6946 CrossRef CAS PubMed; (t) P. Xiao, S. Su, W. Wang, W. Cao, J. Chen, J. Li and Y. Chen, RSC Adv., 2019, 9, 39119 RSC.
- For selected recent studies of our aryne chemistries, see: (a) T. Matsuzawa, K. Uchida, S. Yoshida and T. Hosoya, Org. Lett., 2017, 19, 5521 CrossRef CAS PubMed; (b) T. Matsuzawa, K. Uchida, S. Yoshida and T. Hosoya, Chem. Lett., 2018, 47, 825 CrossRef CAS; (c) S. Yoshida, T. Kuribara, T. Morita, T. Matsuzawa, K. Morimoto, T. Kobayashi and T. Hosoya, RSC Adv., 2018, 8, 21754 RSC; (d) Y. Nishiyama, S. Kamada, S. Yoshida and T. Hosoya, Chem. Lett., 2018, 47, 1216 CrossRef CAS; (e) T. Meguro, S. Chen, K. Kanemoto, S. Yoshida and T. Hosoya, Chem. Lett., 2019, 48, 582 CrossRef CAS; (f) S. Yoshida, Y. Hazama, K. Kanemoto, Y. Nakamura and T. Hosoya, Chem. Lett., 2019, 48, 742 CrossRef CAS; (g) Y. Nakamura, S. Ozawa, S. Yoshida and T. Hosoya, Chem. Lett., 2019, 48, 1296 CrossRef CAS; (h) K. Uchida, Y. Minami, S. Yoshida and T. Hosoya, Org. Lett., 2019, 21, 9019 CrossRef CAS PubMed; (i) T. Kobayashi, T. Hosoya and S. Yoshida, J. Org. Chem., 2020, 85, 4448 CrossRef CAS PubMed; (j) K. Kanemoto, Y. Sakata, T. Hosoya and S. Yoshida, Chem. Lett., 2020, 49, 593 CrossRef CAS.
- For selected transformations of alkynyl sulfides, see: (a) I. D. Gridnev, N. Miyaura and A. Suzuki, J. Org. Chem., 1993, 58, 5351 CrossRef CAS; (b) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2001, 3, 91 CrossRef CAS PubMed; (c) L. Aurelio, R. Volpe, R. Halim, P. J. Scammells and B. L. Flynn, Adv. Synth. Catal., 2014, 356, 1974 CrossRef CAS; (d) L.-G. Xie, S. Shaaban, X. Chen and N. Maulide, Angew. Chem., Int. Ed., 2016, 55, 12864 CrossRef CAS PubMed; (e) R. J. Reddy, M. P. Ball-Jones and P. W. Davies, Angew. Chem., Int. Ed., 2017, 56, 13310 CrossRef CAS PubMed; (f) P. Sharma, R. R. Singh, S. S. Giri, L.-Y. Chen, M.-J. Cheng and R.-S. Liu, Org. Lett., 2019, 21, 5475 CrossRef CAS PubMed; (g) H.-Z. Bu, H.-H. Li, W.-F. Luo, C. Luo, P.-C. Qian and L.-W. Ye, Org. Lett., 2020, 22, 648 CrossRef CAS PubMed; (h) M. B. Reddy and R. Anandhan, Chem. Commun., 2020, 56, 3781 RSC.
- (a) G. R. Ziegler, C. A. Welch, C. E. Orzech, S. Kikkawa and S. I. Miller, J. Am. Chem. Soc., 1963, 85, 1648 CrossRef CAS; (b) M. Arisawa, K. Fujimoto, S. Morinaka and M. Yamaguchi, J. Am. Chem. Soc., 2005, 127, 12226 CrossRef CAS PubMed; (c) R. Frei and J. Waser, J. Am. Chem. Soc., 2013, 135, 9620 CrossRef CAS PubMed; (d) W. Wang, X. Peng, F. Wei, C.-H. Tung and Z. Xu, Angew. Chem., Int. Ed., 2016, 55, 649 CrossRef CAS PubMed; (e) K. Kanemoto, S. Yoshida and T. Hosoya, Org. Lett., 2019, 21, 3172 CrossRef CAS PubMed; (f) W.-C. Gao, Y.-Z. Shang, H.-H. Chang, X. Li, W.-L. Wei, X.-Z. Yu and R. Zhou, Org. Lett., 2019, 21, 6021 CrossRef CAS PubMed.
- See the ESI† for the details.
- (a) J. M. Medina, J. L. Mackey, N. K. Garg and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 15798 CrossRef CAS PubMed; (b) E. Picazo, K. N. Houk and N. K. Garg, Tetrahedron Lett., 2015, 56, 3511 CrossRef CAS PubMed; (c) S. Yoshida, Y. Nakamura, K. Uchida, Y. Hazama and T. Hosoya, Org. Lett., 2016, 18, 6212 CrossRef CAS PubMed.
- Ethyl triflate was not detected in the 1H NMR analysis of the reaction mixture in CD3CN before quenching.
- C. Hall, J. L. Henderson, G. Ernouf and M. F. Greaney, Chem. Commun., 2013, 49, 7602 RSC.
- For selected examples of functionalizations of benzothiophenes, see: (a) A. Krasovskiy, V. Krasovskaya and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 2958 CrossRef CAS PubMed; (b) H. A. Chiong and O. Daugulis, Org. Lett., 2007, 9, 1449 CrossRef CAS PubMed; (c) J.-M. L'Helgoual’ch, A. Seggio, F. Chevallier, M. Yonehara, E. Jeanneau, M. Uchiyama and F. Mongin, J. Org. Chem., 2008, 73, 177 CrossRef PubMed; (d) B. Liégault, D. Lapointe, L. Caron, A. Vlassova and K. Fagnou, J. Org. Chem., 2009, 74, 1826 CrossRef PubMed; (e) T. Liu, X. Shao, Y. Wu and Q. Shen, Angew. Chem., Int. Ed., 2012, 51, 540 CrossRef CAS PubMed; (f) T. Iitsuka, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2015, 80, 2804 CrossRef CAS PubMed; (g) J. M. Hammann, D. Haas and P. Knochel, Angew. Chem., Int. Ed., 2015, 54, 4478 CrossRef CAS PubMed; (h) Z. He, H. J. Shrives, J. A. Fernández-Salas, A. Abengózar, J. Neufeld, K. Yang, A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2018, 57, 5759 CrossRef CAS PubMed; (i) C. Colletto, A. Panigrahi, J. Fernández-Casado and I. Larrosa, J. Am. Chem. Soc., 2018, 140, 9638 CrossRef CAS PubMed; (j) M. Shigeno, K. Hanasaka, K. Sasaki, K. Nozawa-Kumada and Y. Kondo, Chem.–Eur. J., 2019, 25, 3235 CAS; (k) Z.-K. Wen, Z.-K. Zhao, N.-J. Wang, Z.-L. Chen, J.-B. Chao and L.-H. Feng, Org. Lett., 2019, 21, 9545 CrossRef CAS PubMed; (l) J. H. Jang, S. Ahn, S. E. Park, S. Kim, H. R. Byon and J. M. Joo, Org. Lett., 2020, 22, 1280 CrossRef CAS PubMed.
- R. Das and D. Chakraborty, Appl. Organomet. Chem., 2011, 25, 437 CrossRef CAS.
- (a) Y. Zhang, H. Zhao, M. Zhang and W. Su, Angew. Chem., Int. Ed., 2015, 54, 3817 CrossRef CAS PubMed; (b) G. J. P. Perry, J. M. Quibell, A. Panigrahi and I. Larrosa, J. Am. Chem. Soc., 2017, 139, 11527 CrossRef CAS PubMed; (c) J. M. Quibell, G. J. P. Perry, D. M. Cannas and I. Larrosa, Chem. Sci., 2018, 9, 3860 RSC; (d) J.-S. Zhang, T. Chen and L.-B. Han, Eur. J. Org. Chem., 2020, 1148 CrossRef CAS; (e) Y. Wang, B. Li and B. Wang, Org. Lett., 2020, 22, 83 CrossRef CAS PubMed.
- S. Suzuki and J. Yamaguchi, Chem. Commun., 2017, 53, 1568 RSC.
- (a) Y. Liao, Y. Peng, H. Qi, G.-J. Deng, H. Gongac and C.-J. Li, Chem. Commun., 2015, 51, 1031 RSC; (b) H. Qi, Y. Zhang, Q. Tao, J. Chen, Z. Li, X. Xu, H. Tan, Q. Peng and W. Zhu, Tetrahedron, 2016, 72, 7430 CrossRef CAS; (c) H. Qi, X. Xu, Q. Tao, Y. Zhang, M. Zhu, L. Shao, W. Zhu, Q. Peng and Y. Liao, Dyes Pigm., 2017, 142, 406 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization for new compounds including NMR spectra. See DOI: 10.1039/d0sc04450d |
| This journal is © The Royal Society of Chemistry 2020 |