Open Access Article
Open Access ArticleEngineering a highly selective probe for ratiometric imaging of H2Sn and revealing its signaling pathway in fatty liver disease†
Wei
Li‡
a,
Lu
Wang‡
b,
Shulu
Yin
a,
Huanhua
Lai
a,
Lin
Yuan
 *a and
Xiaobing
Zhang
*a and
Xiaobing
Zhang
 a
a
aState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China. E-mail: lyuan@hnu.edu.cn
bDepartment of Chemical Biology, Max Planck Institute for Medical Research, Jahnstrasse 29, Heidelberg 69120, Germany
First published on 9th July 2020
Abstract
Hydrogen polysulfides (H2Sn, n > 1) have continuously been proved to act as important signal mediators in many physiological processes. However, the physiological role of H2Sn and their signaling pathways in complex diseases, such as the most common liver disease, nonalcoholic fatty liver disease (NAFLD), have not been elucidated due to lack of suitable tools for selective detection of intracellular H2Sn. Herein, we adopted a general and practical strategy including recognition site screening, construction of a ratiometric probe and self-assembly of nanoparticles, to significantly improve the probes' selectivity, photostability and biocompatibility. The ratiometric probe PPG-Np-RhPhCO selectively responds to H2Sn, avoiding interaction with biothiol and persulfide. Moreover, this probe was applied to image H2Sn in NAFLD for the first time and reveal the H2Sn generation pathways in the cell model of drug-treated NAFLD. The pathway of H2Sn revealed by PPG-Np-RhPhCO provides significant insights into the roles of H2Sn in NAFLD and future drug development.
Introduction
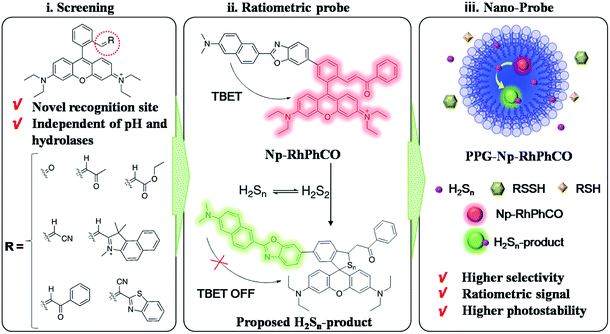
Hydrogen sulfide, one of the reactive sulfur species (RSS), has proved to be a key molecule in many diseases.1,2 Studies have shown that H2S and H2Sn are redox partners in terms of chemical properties and coexist in biological systems.3,4 Recently, accumulated data have indicated the important roles of H2Sn in signal transduction in physiological processes, such as cardiovascular system, ion channels, oxidative posttranslational modification, antioxidation and cytoprotection.5–11 Furthermore, aberrant production and physiological levels of H2Sn are found to be correlated with physiological processes and diseases such as sulfur redox balance and liver disease.12,13 Nonalcoholic fatty liver disease (NAFLD), referring to a wide spectrum of liver damage that ranges from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis, represents the most common chronic liver disease in developed countries. Clarification of the functions of signaling molecules in NAFLD offers important insights into the pathogenesis of the disease, drug development and therapeutic evaluation.14,15 Even though some research studies have implied the existence of H2Sn in liver disease,12,16,17 the role and signaling pathway of H2Sn in the process of NAFLD have not been revealed due to the lack of suitable analytical methods for selectively monitoring H2Sn in living biological systems.Some in vitro analysis techniques including UV-spectroscopy, cold cyanolysis and mass spectrometry have been described to detect H2Sn,5,18 while fluorescence imaging has received widespread attention in H2Sn bioanalysis due to its unique advantages in live cell imaging, such as excellent biocompatibility and high spatial and temporal resolution.19 Previous efforts in designing H2Sn fluorescent probes were mainly based on conjugating functional groups, such as 2-fluoro-5-nitro-benzoic ester, phenyl-2-(benzoylthio)benzoate, and cinnamate ester, with hydroxyl-containing fluorophores.4,20,21 However, these functional groups may also react with abundant biothiols and active hydrolases inside cells, which could cause interference in H2Sn detection. Only a few probes have been designed based on other electrophilic receptors, such as derivatives of xanthene or silicon-rhodamines.22–24 However, there are currently no methods suitable for selectively sensing H2Sn from other persulfides (RSSH). At the same time, poor solubility, concentration fluctuation and unsatisfactory photostability make it more difficult to accurately monitor the changes of H2Sn in live-cell imaging. To address these problems, we report a first-generation through-bond energy transfer (TBET) ratiometric fluorescent probe, PPG-Np-RhPhCO, with significantly improved photostability, selectivity and biocompatibility for monitoring intracellular H2Snvia a simple and general strategy. The design concept consists of three steps (Fig. 1): (i) screening of new recognition sites based on the stable electrophilic receptor, enabling an optimal response site for specific detection of H2Sn that is independent of pH and hydrolases; (ii) construction of ratiometric probes, achieving accurate detection of H2Sn by single-wavelength excitation; (iii) self-assembly of nanoparticles, improving the selectivity, photostability and water solubility. The nano-probe PPG-Np-RhPhCO was successfully applied for monitoring the generation of H2Sn in NAFLD, which to our knowledge is reported for the first time. Importantly, we also show that 3-mercaptopyruvate sulfurtransferase (MPST) and cystathionine γ-lyase (CSE) are largely responsible for participating in the regulation of H2Sn. Furthermore, we revealed that ROS/H2S/H2Sn crosstalk signaling pathways that increased ROS in the drug-treated NAFLD model would stimulate MPST and CSE to produce more H2S, which causes significant enhancement of H2Snvia reaction with ROS or catalysis by MPST and CSE.
Results and discussion
Design and screening of recognition sites
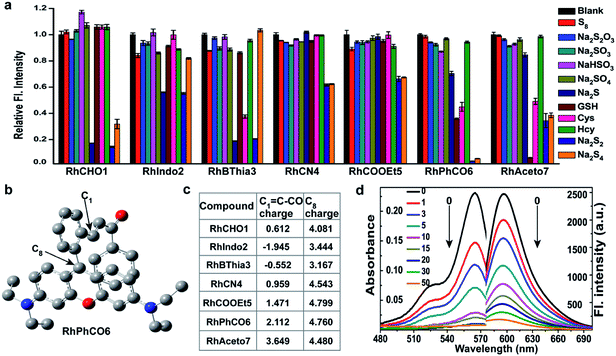
Although several fluorescent probes for H2Sn were prepared based on the introduction of aromatic ester groups into fluorophores,12,20,21,25,26 their poor stability resulting from the susceptibility to pH and hydrolase could easily cause false signals, thereby limiting their application in live-cell imaging. Several persulfide probes based on xanthene derivatives that are susceptible to nucleophilic attack and regulate the fluorescence changes for hydropolysulfide detection have been reported,22–24 but they lack the specificity of H2Sn. To address these concerns, unsaturated double bonds containing different electron-withdrawing groups were introduced into the benzene moiety of xanthene dyes (Fig. 1 and Scheme S1†). It was proposed that the reaction with H2Sn produces persulfide intermediates via nucleophilic addition and then rapidly undergo intramolecular spirocyclization because of the high nucleophilicity and bis-nucleophiles of persulfide intermediates.27 As a proof of concept, seven xanthene derivatives bearing different substituents containing unsaturated double bonds (RhCHO1, RhIndo2, RhThia3, RhCN4, RhCOOEt5, RhPhCO6, and RhAton7 in Scheme S2†) were synthesized and tested as candidates for new recognition site screening. To examine the fluorescence response toward different RSS, S8, S2O32−, SO32−, HSO3−, SO42−, Na2S, GSH, Cys, Hcy, Na2S2 and Na2S4 were incubated with the probe in phosphate buffer (25 mM PBS mixed with 1% MeCN, pH 7.4) separately (Fig. 2, S1 and S2†). RhPhCO6 was chosen as the target recognition site due to fast response to H2Sn (<1 min, Fig. S3a†) and resistance to pH (pH 4.0–11.0, Fig. S3b†). The higher reaction activity of RhPhCO6 toward H2Sn than of the other six compounds was further explained by density functional theory (DFT) calculations (Fig. 2 and S4†), and it was a result of proper electrostatic charges of the β-carbon of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the medial carbon of rhodamine in RhPhCO6, as well as steric hindrance. The reaction between unsaturated double bonds and H2Sn was followed by rapid intramolecular spirocyclization (Fig. S5†), as evidenced in the high resolution mass spectrum (Fig. S6†) and 1H NMR spectrum (Fig. S7†). Furthermore, to examine the possible reasons for the specific response of RhPhCO6 towards H2Sn rather than H2S, time-dependent 1H NMR spectra of the reaction between RhPhCO6 and H2Sn or H2S were recorded (Fig. S7 and S8†). The excellent selectivity is due to the ultra-fast reaction between RhPhCO6 and H2Sn, which is probably caused by the special alpha effect of H2Sn.18,28 Even novel recognition sites in RhPhCO6 exhibit excellent response towards H2Sn, but a reaction with a high concentration of GSH and Cys could cause some interference with intracellular H2Sn sensing. Therefore, the self-assembly strategy of amphiphilic polymers was further utilized to increase the selectivity, which will be detailed in the next paragraph.
C bond and the medial carbon of rhodamine in RhPhCO6, as well as steric hindrance. The reaction between unsaturated double bonds and H2Sn was followed by rapid intramolecular spirocyclization (Fig. S5†), as evidenced in the high resolution mass spectrum (Fig. S6†) and 1H NMR spectrum (Fig. S7†). Furthermore, to examine the possible reasons for the specific response of RhPhCO6 towards H2Sn rather than H2S, time-dependent 1H NMR spectra of the reaction between RhPhCO6 and H2Sn or H2S were recorded (Fig. S7 and S8†). The excellent selectivity is due to the ultra-fast reaction between RhPhCO6 and H2Sn, which is probably caused by the special alpha effect of H2Sn.18,28 Even novel recognition sites in RhPhCO6 exhibit excellent response towards H2Sn, but a reaction with a high concentration of GSH and Cys could cause some interference with intracellular H2Sn sensing. Therefore, the self-assembly strategy of amphiphilic polymers was further utilized to increase the selectivity, which will be detailed in the next paragraph.
Construction of the ratiometric fluorescent nano-probe
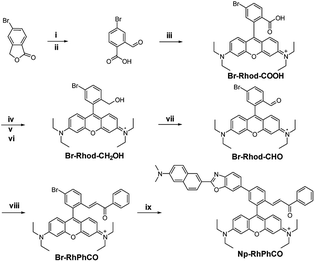
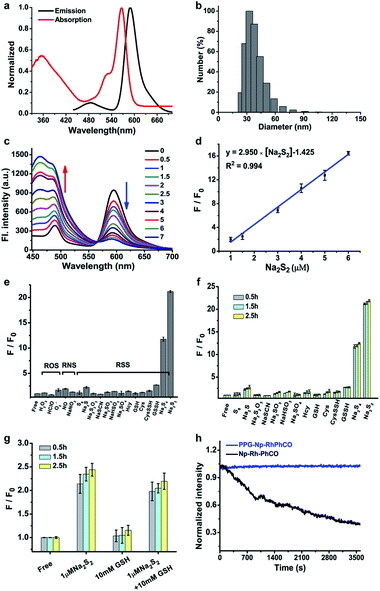
The ratiometric signal offers more accurate detection of intracellular analytes due to its resistance to the influence of probe concentration and various cellular environments.29–31 Several ratiometric probes for H2Sn have been reported recently, whose ratiometric mechanisms were achieved by intramolecular charge transfer (ICT) and Förster resonance energy transfer (FRET).12,22,32 Ratiometric probes based on through-bond energy transfer (TBET) usually exhibit higher energy transfer efficiency than those based on FRET even without spectral overlap.33 Thus, a ratiometric fluorescent probe Np-RhPhCO for sensing H2Sn was constructed by combining a naphthalene unit (donor) and RhPhCO6 (acceptor) via Through-Bond Energy Transfer (TBET) cassettes (Scheme 1). The synthesis of probe Np-RhPhCO started from commercially available 5-bromophthalide using bromination and condensation to yield Br-Rhod-COOH and then reduction, oxidation and alcohol oxidation to obtain Br-Rhod-CHO (Scheme 1 and S3†). And the receptor Br-RhPhCO was subsequently synthesized via the classic Wittig reaction. Lastly, Pd-catalyzed Negishi cross-coupling of Br-RhPhCO and the boronic acid pinacol ester of Np (Np-Borate) provided the TBET-based ratiometric probe Np-RhPhCO (synthesis and characterization details in the ESI†). As reported before,34 the naphthalene–rhodamine TBET platform showed two well-separated absorbance peaks at 355 nm and 565 nm with emission peaks at 486 nm and 591 nm, respectively (Fig. 3a). Titration of Na2S2 (0–50 μM) gradually increased the fluorescence at 486 nm and decreased the fluorescence at 591 nm, offering a 59-fold fluorescence ratio (I486/I591) enhancement (Fig. S9†).In recent years, nanoparticles used to improve the photostability and biocompatibility of small organic molecules have attracted increasing attention in the fields of biosensing, in vivo imaging and drug release.35–38 In addition, it has been reported that the selectivity can be improved by encapsulating an organic fluorescent probe in a self-assembled supramolecular system of an amphiphilic polymer.39 In order to achieve the application in a complex living system, we tried to encapsulate the small molecule ratiometric probe Np-RhPhCO into the self-assembled nanoparticles based on an amphiphilic copolymer to construct a more suitable organic nano-probe. 1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) and poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (mPEG-PPG-PEG) were applied to prepare the nano-probes, respectively (Fig. S10–13†). Among them, PPG-Np-RhPhCO with a 33 nm average hydrodynamic size (Fig. 3b and S12b†) exhibited a 21-fold ratiometric signal (I486/I591) enhancement in the presence of H2Sn (Fig. 3c and d), displaying a better response than DSPE-Np-RhPhCO. The detection limit was calculated to be as low as 9.4 nM, indicating the great potential to detect endogenous H2Sn in live cells. Importantly, 7 μM Na2S2 triggered a prominent enhancement of the fluorescence intensity ratio (I486/I591), while negligible signal fluctuation was obtained in the presence of other analytes (30 μM to 1 mM) (Fig. 3e). Notably, compared with Np-RhPhCO, the nanoprobe PPG-Np-RhPhCO showed less response towards bulky biothiols and their hydropolysulfides (Cys, GSH, CysSSH and GSSH) even with a longer incubation time (2.5 h) (Fig. 3f and g), which is probably due to physical blocking of the interaction. In addition, PPG-Np-RhPhCO is capable of responding toward H2Sn over a wide pH range (pH 4.0–11.0) (Fig. S12a†).
Another key parameter of ideal fluorescent probes for living cell imaging is stability, primarily including long-term stability and photostability. The formed nano-probe showed good stability with only slight spectral changes for ten days at room temperature (Fig. S13†). Furthermore, illumination with continuous laser scanning showed that the fluorescence signal of PPG-Np-RhPhCO remained stable over 3600 s, while the intensity of the parent probe Np-RhPhCO decreased by 62 percent, demonstrating the significantly improved photostability of the nano-probe (Fig. 3h). This was ascribed to the fact that the outer polymer micelle provides an effective shelter protecting the inner organic small molecule probe Np-RhPhCO from the surrounding environment.40
Monitoring endogenous H2Sn generation in live cells
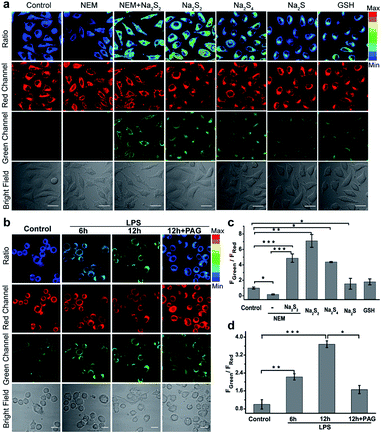
Inspired by the excellent in vitro response, we then investigated the performance of PPG-Np-RhPhCO for detecting endogenous generation of H2Sn in live cells. The cytotoxicity of PPG-Np-RhPhCO in cells was first examined by standard MTT assays, indicating negligible cytotoxicity on the human liver cell line HL-7702 (L02) after 24 h of incubation (Fig. S14†). In good agreement with the in vitro photostability test, the probe PPG-Np-RhPhCO exhibited significantly higher photostability than the parent small-molecule probe Np-RhPhCO in live cells under continuous excitation (Fig. S15†). To demonstrate the capability of PPG-Np-RhPhCO for detecting endogenous H2Sn, we next performed live-cell imaging under different stimulation conditions. As shown in Fig. 4a and c, L02 cells incubated with PPG-Np-RhPhCO showed weak fluorescence in the green channel (425–475 nm) but strong fluorescence in the red channel (570–620 nm) in the cytosol, indicating a low level of H2Sn in the normal state. A lower FGreen/FRed value was observed when 500 μM N-ethylmaleimide (NEM, a RSS blocking agent32) was pretreated for 30 min to cause the depletion of endogenous polysulfide and mercapto compounds. In contrast, addition of Na2S2 (30 μM) or Na2S4 (30 μM), donors of H2Sn, causes a significant ratiometric signal increase. Notably, 50 μM Na2S and 1 mM GSH provide only slight FGreen/FRed changes, demonstrating the high selectivity of PPG-Np-RhPhCO towards H2Sn in living cells. Next, we further examined the capability of the nano-probe for detecting endogenous H2Sn generation in RAW 264.7 macrophages. It has been reported that bacterial endotoxin lipopolysaccharide (LPS) can induce the over-expression of cystathionine γ-lyase (CSE), which mediated cysteine metabolism for H2Sn formation in RAW 264.7 macrophages.41 Thus, we treated RAW 264.7 macrophages with the probe PPG-Np-RhPhCO in the presence of LPS with different incubation times (Fig. 4b and d). It was shown that the fluorescence ratio (FGreen/FRed) gradually increased as a function of incubation time, while introduction of 1 mM DL-propargylglycine (PAG, a commercial CSE irreversible inhibitor42) yielded a clearly reduced fluorescence ratio signal. Moreover, the capability of the nano-probe PPG-Np-RhPhCO for imaging H2Sn was further confirmed via two-photon imaging in live cells, zebrafish, and fresh liver tissues (Fig. S16 and S17†).H2S and MPST involved endogenous H2Sn generation in NAFLD
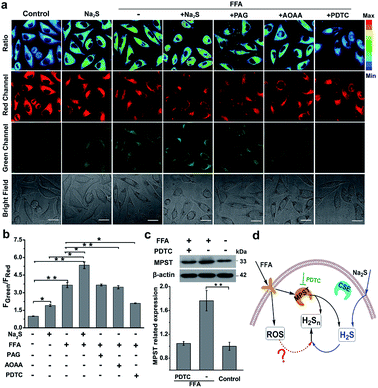
Nonalcoholic fatty liver disease (NAFLD) is a common type of liver disease that evolves from simple steatosis to hepatitis and liver cancer. Determination of signaling molecules in the process of NAFLD offers insights into disease prevention, drug development and therapeutic evaluation.14 H2S has been studied for decades to elucidate its functions in NAFLD;43,44 however, H2Sn, as the partner of H2S coexisting in the organism, has not been investigated in NAFLD due to the lack of suitable probes. Herein, we tried to examine the existence of H2Sn in NAFLD and further reveal the possible pathway of H2Sn generation. The NAFLD model was produced via incubating normal liver L02 cells with 0.5 mM FFA for 12 h,45 which was confirmed by the significantly increased lipid droplets and triglycerides based on Oil Red O staining experiments and triglyceride test kits (Fig. S18†). As shown in Fig. 5a and b, treatment with FFA leads to a huge increase in the ratiometric signal (FGreen/FRed = 3.9), indicating the high level of H2Sn in NAFLD. Interestingly, an increase of the fluorescence ratio was observed in both normal L02 cells and FFA-stimulated L02 cells when a higher concentration of Na2S (the donor of H2S) was produced, indicating that the key role of H2S is the regulation of endogenous H2Sn (Fig. 5d). Moreover, a higher level of endogenous H2S in NFLAD was observed after staining with the reported H2S probe TPC-N3 (Fig. S19†),46 which further suggests a positive correlation between H2S and H2Sn production in NAFLD.Given that both H2S and H2Sn can be produced from three major enzymes, CSE, cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (MPST),47–49 the roles of these enzymes in the regulation of production of H2Sn in FFA-induced NAFLD were examined. As shown in Fig. 5a, a significant reduction of the fluorescence ratio was noted upon addition of pyrrolidine dithiocarbamate (PDTC), which reduced MPST expression by inhibiting the nuclear factor NF-κB/p65.45 These results were further confirmed by western blot analysis (Fig. 5c). However, pretreatment with PAG (a CSE inhibitor) and aminooxyacetic acid (AOAA, a CBS inhibitor42) displayed a negligible change of the fluorescence ratio (Fig. 5a). Therefore, we assumed that MPST participates in the regulation of H2Sn production in the NAFLD model. The participation of MPST in the regulation of H2S production under the treatment of FFA was also observed in Li's work.45 Taken together, we assumed that addition of Na2S can increase H2S, the precursor of H2Sn, thus promoting the further increase of H2Sn in the NAFLD model. Meanwhile, up-regulation of the expression of MPST stimulated by FFA can also promote the generation of H2Sn by producing more H2S or direct reaction with H2S (Fig. 5d).
ROS/H2S/H2Sn crosstalk signaling pathways in the NAFLD model
Previous literature showed that the reactive oxygen species (ROS) level in NAFLD increases greatly due to the progressive increase of oxidative stress levels caused by mitochondrial abnormalities in hepatic cells.50 In addition, it is reported that ROS and RNS (including HClO, H2O2, O2˙−, NO, ONOO−, etc.) react with H2S to form H2Sn, RSS−, SO42−, SO32−, and S2O32−;10,51,52 thus we assumed that higher ROS levels in the NAFLD model would also contribute to production and regulation of H2Sn (Fig. 5d). Moreover, there is growing evidence that long-term drug use for NAFLD patients could aggravate NAFLD and further increase the level of intracellular ROS.53–56 Thus, a commonly used drug, acetaminophen (APAP, also known as paracetamol), was chosen to further elucidate the potential pathways of H2Sn production in a drug-treated NAFLD model.56,57 As shown in Fig. S21,† both FFA and APAP can lead to higher levels of ONOO−, one of the representative ROS, which was indicated by the reported probe MITO-CC.58 It was shown that co-treatment with APAP and FFA causes higher ROS production than individual treatment separately (Fig. S20†), probably due to the aggravation of oxidative stress caused by the synergistic effect of APAP and FFA.56 Significantly, consistent enhancement of H2Sn and ROS under the above conditions was clearly observed (Fig. 6 and S21†), strongly supporting our assumption that ROS plays an important role in the regulation of H2Sn production. We also investigated the change of the H2Sn level via two-photon tissue imaging of APAP-treated NAFLD mice, which was consistent with the results of cells imaging (Fig. S22†).Since we have revealed the function of H2S in regulation of H2Sn generation in NAFLD, we next examined the H2S level changes in the presence of a high concentration of ROS stimulated by APAP and FFA. Interestingly, consistent with ROS level changes, the H2S level also increased more with co-treatment with FFA and APAP than when treated with FFA or APAP alone (Fig. 6d, S19 and S20†). The consistent variation between ROS and H2S is probably because of the redox homeostasis in living cells.59–61 Moreover, treatment with the antioxidant ascorbic acid (VC, a natural, effective antioxidant62) in NAFLD greatly reduced H2Sn, H2S and ROS levels simultaneously. Taken together, we assumed that direct reaction between H2S and ROS is probably one of main pathways for producing H2Sn, forming a ROS/H2S/H2Sn crosstalk signaling pathway.
To further explore the functions of enzymes CSE, MPST and CBS in regulating the ROS/H2S/H2Sn pathway, PAG (inhibitor of CSE), PDTC (inhibitor of MPST), and AOAA (inhibitor of CBS) were treated, respectively, prior to imaging H2Sn in APAP and FFA-treated L02 cells (Fig. 6a and b). Both H2Sn and H2S displayed a significant decrease in the presence of PAG or PDTC, which points to the capability of CSE and MPST in regulating H2Sn and H2S in the NAFLD model. However, the inhibitor AOAA had no obvious inhibitory effects maybe because the abundance of CBS in the liver is much lower than that of CSE.63 These results were also consistent with the CSE and MPST western blot analysis results (Fig. 6c). Thus, the consistent changes of H2S and H2Sn by regulation of CSE and MPST allow us to confirm the roles of H2S, CSE and MPST in the H2Sn generation pathways (Fig. 6e). In combination with the participation of ROS in regulation of H2Sn generation, we proposed the ROS/H2S/H2Sn crosstalk signaling pathways in Fig. 6e. Increased ROS in FFA-induced NAFLD stimulates MPST and CSE to produce more H2S, which causes enhancement of H2Snvia direct reaction with ROS or catalysis by MPST and CSE.
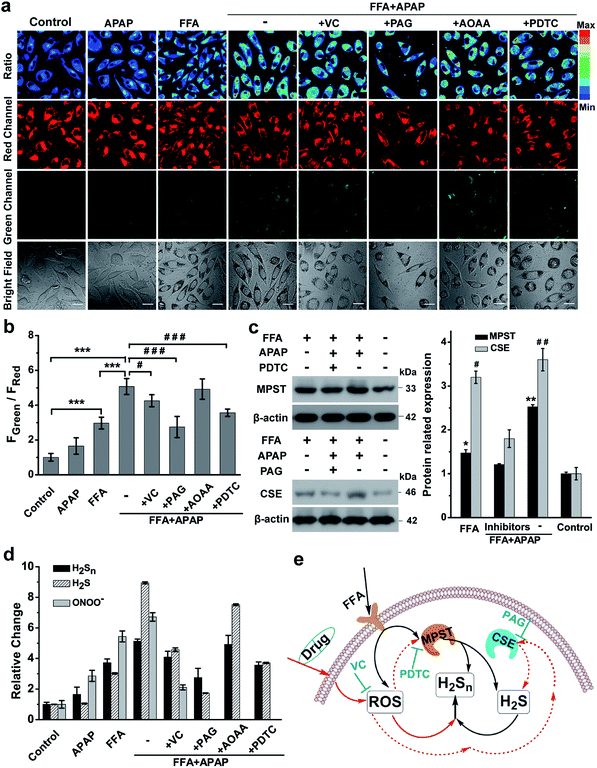 | ||
| Fig. 6 H2Sn imaging in drug-treated NAFLD. (a) Confocal fluorescence images of the nano-probe PPG-Np-RhPhCO (4.8 μM) in L02 cells under different conditions. The first column: incubation with the nano-probe for 2 h prior to imaging; the second and third columns: incubation with the nano-probe for 2 h after treatment with APAP (3 mM) or FFA (0.5 mM) for 12 h, respectively. The fourth to eighth columns: treatment with FFA (0.5 mM) for 12 h and VC (1 mM), PAG (1 mM), AOAA (300 μM) or PDTC (200 μM) for 2 h, respectively, and then with APAP (3 mM) for 12 h and with the nano-probe for 2 h. Green channel (425–475 nm), red channel (570–620 nm), pseudocolor ratio images (green channel/red channel). λex = 405 nm. Scale bar: 20 μm. (b) Average fluorescence intensity ratios (FGreen/FRed) of live L02 cells under the conditions in (a). Data represent mean standard error (n = 5 independent experiments; 80 cells). Statistical significance in (a) was calculated with unpaired two-tailed Student's t-tests. ***p < 0.001. Significance is expressed as # compare to the co-treatment with FFA and APAP, #p < 0.05, ###p < 0.001. (c) Western blot analysis showing MPST and CSE expression in L02 cells upon treatment with FFA, APAP and inhibitor (PDTC or PAG), respectively. The MPST and CSE relative abundance was normalized with β-actin relative abundance. n = 3 independent experiments. (d) The relative levels of ONOO−, H2S and H2Sn in the NAFLD model. The data were based on fluorescence imaging of L02 cells stained with ONOO−, H2S and H2Sn probes (MITO-CC in Fig. S21,† TPC-N3 in Fig. S20† and PPG-Np-RhPhCO in Fig. 6a), respectively, and were normalized with their respective control group. (e) Schematic of ROS/H2S/H2Sn crosstalk signaling pathways that regulate H2Sn generation in the NAFLD model. Increased ROS in FFA-induced NAFLD stimulate MPST and CSE to produce more H2S, which causes enhancement of H2Snvia direct reaction with ROS or catalysis by MPST and CSE. Black arrows denote the H2Sn generation pathway in FFA-induced NAFLD; red solid arrows and dashed arrows indicate ROS roles in the regulation of H2Sn in drug-treated NAFLD, respectively. VC represents antioxidants while PDTC and PAG indicate MPST and CSE inhibitors, respectively. | ||
In this work, to accurately and selectively image the intracellular H2Sn, we introduced a new ratiometric fluorescent probe PPG-Np-RhPhCO, characterized by three main features that distinguish it from previous H2Sn sensors. First, rational design and fast screening offer new recognition sites for sensing H2Sn, which avoid interference of pH and hydrolases in living cells. Second, introduction of nanoparticles formed by polymers enables the further improvement of selectivity of the probe, which is a prerequisite for applications in complex biological environments and a bottleneck for most small-molecule fluorescent probes. The encapsulation of fluorophores into nanoparticles works as a general method to improve the selectivity because it avoids the interaction with bulky potential competitors (Cys, GSH, CysSSH and GSSH), which were main interferences for previous H2Sn probes. Third, the nano-probe can offer more accurate detection of H2Sn through ratiometric imaging in living cells. The ratiometric quantification calculated from two signal channels can exclude the interference of probe concentration, which particularly benefits cell imaging under different stimulation conditions.
To examine the application of the new H2Sn probe in live-cell imaging, we incubated PPG-Np-RhPhCO with the liver cell line, HL-7702 cells, and murine macrophage cell line, RAW 264.7 cells. Live-cell imaging demonstrated the capability of the probe in detecting endogenous H2Sn with good sensitivity and signal to background ratio (Fig. 4). The excellent performance of this probe in imaging and monitoring H2Sn inspired us to address the challenge of H2Sn production pathways. In the NAFLD model, we found that FFA could stimulate MPST expression, which promotes the generation of H2Sn. Moreover, as the precursor, H2S at higher concentration can promote the further increase of H2Sn in the NAFLD model. To further explore the roles of ROS in the production of H2Sn, we introduced a widely used drug, APAP, to boost ROS production in the NAFLD model. Simultaneous treatment with APAP and FFA causes a dramatic increase of ROS, which stimulates MPST and CSE to increase the generation of H2S to maintain redox homeostasis. It is also worth noting that higher expression of CSE in L02 cells was observed under the treatment with FFA and APAP simultaneously, but not under treatment with FFA alone. This is probably because ROS at very high levels can stimulate CSE up-regulation to elevate the level of antioxidant H2S to maintain the redox balance.64 A higher level of ROS and H2S can generate more H2Snvia chemical reaction, forming one of main pathways for H2Sn production in NAFLD. Meanwhile, MPST and CSE which are highly expressed under FFA and APAP-stimulation can also directly induce H2S to generate H2Sn, forming another main pathway for H2Sn production in NAFLD. The ROS/H2S/H2Sn pathways in the NAFLD model (Fig. 6e) reported in this work will provide new insights into future drug development and therapeutic evaluation for NAFLD patients.
Conclusions
In summary, we designed and developed a ratiometric fluorescent probe, PPG-Np-RhPhCO, for monitoring the generation of endogenous H2Sn in NAFLD. The new probe, PPG-Np-RhPhCO, was successfully applied to reveal the possible ROS/H2S/H2Sn pathways in the NAFLD model. The excellent selectivity, photostability and biocompatibility of the probe PPG-Np-RhPhCO enable it to be a practical and direct tool for detecting and studying intracellular H2Sn in complex biological samples.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Hunan University, and all animal experiments were approved by the Animal Ethics Committee of the College of Biology (Hunan University).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (21877029, 21735001, and 21622504) and the National Key R&D Program of China (2019YFA0210103). We thank Dr W. Chen and Prof. M. Xian (Washington State University) for supplying Na2S2 and Na2S4.Notes and references
- M. R. Filipovic, J. Zivanovic, B. Alvarez and R. Banerjee, Chem. Rev., 2017, 118, 1253–1337 CrossRef PubMed.
- M. Yang, J. Fan, J. Du and X. Peng, Chem. Sci., 2020, 11, 5127–5141 RSC.
- G. I. Giles, K. M. Tasker and C. Jacob, Free Radical Biol. Med., 2001, 31, 1279–1283 CrossRef CAS PubMed.
- C. Liu, W. Chen, W. Shi, B. Peng, Y. Zhao, H. Ma and M. Xian, J. Am. Chem. Soc., 2014, 136, 7257–7260 CrossRef CAS PubMed.
- R. Greiner, Z. Pálinkás, K. Bäsell, D. Becher, H. Antelmann, P. Nagy and T. P. Dick, Antioxid. Redox Signaling, 2013, 19, 1749–1765 CrossRef CAS PubMed.
- H. Kimura, Proc. Jpn. Acad., Ser. B, 2015, 91, 131–159 CrossRef CAS PubMed.
- H. Kimura, Nitric Oxide, 2014, 41, 4–10 CrossRef CAS PubMed.
- D. Zhang, I. Macinkovic, N. O. Devarie-Baez, J. Pan, C. M. Park, K. S. Carroll, M. R. Filipovic and M. Xian, Angew. Chem., Int. Ed., 2014, 53, 575–581 CrossRef CAS PubMed.
- H. Kimura, Molecules, 2014, 19, 16146 CrossRef PubMed.
- K. Hideo, Antioxid. Redox Signaling, 2017, 27, 619–621 CrossRef PubMed.
- M. C. Gruhlke and A. J. Slusarenko, Plant Physiol. Biochem., 2012, 59, 98–107 CrossRef CAS PubMed.
- J. Zhang, X. Y. Zhu, X. X. Hu, H. W. Liu, J. Li, L. L. Feng, X. Yin, X. B. Zhang and W. Tan, Anal. Chem., 2016, 88, 11892–11899 CrossRef CAS PubMed.
- N. E. Francoleon, S. J. Carrington and J. M. Fukuto, Arch. Biochem. Biophys., 2011, 516, 146–153 CrossRef CAS PubMed.
- N. Berndt, S. Bulik, I. Wallach, T. Wünsch, M. König, M. Stockmann, D. Meierhofer and H. G. Holzhütter, Nat. Commun., 2018, 9, 2386 CrossRef PubMed.
- S. K. Erickson, J. Lipid Res., 2009, 50, S412–S416 CrossRef PubMed.
- K. Namekata, Y. Enokido, I. Ishii, Y. Nagai, T. Harada and H. Kimura, J. Biol. Chem., 2004, 279, 52961–52969 CrossRef CAS PubMed.
- S. Mani, G. Yang and R. Wang, Free Radical Biol. Med., 2011, 50, 1280–1287 CrossRef CAS PubMed.
- C. M. Park, L. Weerasinghe, J. J. Day, J. M. Fukuto and M. Xian, Mol. BioSyst., 2015, 11, 1775–1785 RSC.
- P. Gao, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 6035–6071 RSC.
- W. Chen, E. W. Rosser, T. Matsunaga, A. Pacheco, T. Akaike and M. Xian, Angew. Chem., Int. Ed., 2015, 54, 13961–13965 CrossRef CAS PubMed.
- Y. Hou, X. F. Yang, Y. Zhong and Z. Li, Sens. Actuators, B, 2016, 232, 531–537 CrossRef CAS.
- K. Umezawa, M. Kamiya and Y. Urano, Angew. Chem., Int. Ed., 2018, 57, 9346–9350 CrossRef CAS PubMed.
- R. Kawagoe, I. Takashima, S. Uchinomiya and A. Ojida, Chem. Sci., 2017, 8, 1134–1140 RSC.
- Y. Takano, K. Hanaoka, K. Shimamoto, R. Miyamoto, T. Komatsu, T. Ueno, T. Terai, H. Kimura, T. Nagano and Y. Urano, Chem. Commun., 2017, 53, 1064–1067 RSC.
- F. Yu, M. Gao, M. Li and L. Chen, Biomaterials, 2015, 63, 93–101 CrossRef CAS PubMed.
- L. Zeng, S. Chen, T. Xia, W. Hu, C. Li and Z. Liu, Anal. Chem., 2015, 87, 3004–3010 CrossRef CAS PubMed.
- E. Cuevasanta, M. N. Moller and B. Alvarez, Arch. Biochem. Biophys., 2017, 617, 9–25 CrossRef CAS PubMed.
- E. Cuevasanta, M. Lange, J. Bonanata, E. L. Coitino, G. Ferrer-Sueta, M. R. Filipovic and B. Alvarez, J. Biol. Chem., 2015, 290, 26866–26880 CrossRef CAS PubMed.
- L. Yuan, W. Lin, K. Zheng and S. Zhu, Acc. Chem. Res., 2013, 46, 1462–1473 CrossRef CAS PubMed.
- M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185–4191 RSC.
- W. Li, S. Yin, X. Gong, W. Xu, R. Yang, Y. Wan, L. Yuan and X. B. Zhang, Chem. Commun., 2020, 56, 1349–1352 RSC.
- Q. Han, Z. Mou, H. Wang, X. Tang, Z. Dong, L. Wang, X. Dong and W. Liu, Anal. Chem., 2016, 88, 7206–7212 CrossRef CAS PubMed.
- W. Lin, L. Yuan, Z. Cao, Y. Feng and J. Song, Angew. Chem., Int. Ed., 2010, 49, 375–379 CrossRef CAS PubMed.
- L. Zhou, X. Zhang, Q. Wang, Y. Lv, G. Mao, A. Luo, Y. Wu, Y. Wu, J. Zhang and W. Tan, J. Am. Chem. Soc., 2014, 136, 9838–9841 CrossRef CAS PubMed.
- M. De, P. S. Ghosh and V. M. Rotello, Adv. Mater., 2008, 20, 4225–4241 CrossRef CAS.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79–93 RSC.
- L. Chen, S. Xu, W. Li, T. Ren, L. Yuan, S. Zhang and X. B. Zhang, Chem. Sci., 2019, 10, 9351–9357 RSC.
- L. Wu, Y. Sun, K. Sugimoto, Z. Luo, Y. Ishigaki, K. Pu, T. Suzuki, H. Y. Chen and D. Ye, J. Am. Chem. Soc., 2018, 140, 16340–16352 CrossRef CAS PubMed.
- J. Yan, M. C. Estévez, J. E. Smith, K. Wang, X. He, L. Wang and W. Tan, Nano Today, 2007, 2, 44–50 CrossRef.
- X. Y. Zhu, S. J. Liu, Y. J. Liu, S. Wang and X. Ni, Cell. Mol. Life Sci., 2010, 67, 1119–1132 CrossRef CAS PubMed.
- A. Asimakopoulou, P. Panopoulos, C. T. Chasapis, C. Coletta, Z. Zhou, G. Cirino, A. Giannis, C. Szabo, G. A. Spyroulias and A. Papapetropoulos, Br. J. Pharmacol., 2013, 169, 922–932 CrossRef CAS PubMed.
- Z. L. Luo, L. J. Tang, T. Wang, R. W. Dai, J. D. Ren, L. Cheng, K. Xiang and F. Z. Tian, J. Gastroenterol. Hepatol., 2014, 29, 215–222 CrossRef CAS PubMed.
- K. S. Lindsei, L. S. Yaw and O. Karmin, Can. J. Physiol. Pharmacol., 2015, 93, 1–11 CrossRef PubMed.
- M. Li, C. Xu, J. Shi, J. Ding, X. Wan, D. Chen, J. Gao, C. Li, J. Zhang, Y. Lin, Z. Tu, X. Kong, Y. Li and C. Yu, Gut, 2018, 67, 2169–2180 CrossRef CAS PubMed.
- T. B. Ren, W. Xu, Q. L. Zhang, X. X. Zhang, S. Y. Wen, H. B. Yi, L. Yuan and X. B. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7473–7477 CrossRef CAS PubMed.
- S. Yuan, X. Shen and C. G. Kevil, Antioxid. Redox Signaling, 2017, 27, 634–653 CrossRef CAS PubMed.
- N. Shibuya, M. Tanaka, M. Yoshida, Y. Ogasawara, T. Togawa, K. Ishii and H. Kimura, Antioxid. Redox Signaling, 2008, 11, 703–714 CrossRef PubMed.
- T. Ida, T. Sawa, H. Ihara, Y. Tsuchiya, Y. Watanabe, Y. Kumagai, M. Suematsu, H. Motohashi, S. Fujii, T. Matsunaga, M. Yamamoto, K. Ono, N. O. Devarie-Baez, M. Xian, J. M. Fukuto and T. Akaike, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7606–7611 CrossRef CAS PubMed.
- A. Borrelli, P. Bonelli, F. M. Tuccillo, I. D. Goldfine, J. L. Evans, F. M. Buonaguro and A. Mancini, Redox Biol., 2018, 15, 467–479 CrossRef CAS PubMed.
- B. L. Predmore, D. J. Lefer and G. Gojon, Antioxid. Redox Signaling, 2012, 17, 119–140 CrossRef CAS PubMed.
- M. M. Cortese-Krott, G. G. C. Kuhnle, A. Dyson, B. O. Fernandez, M. Grman, J. F. DuMond, M. P. Barrow, G. McLeod, H. Nakagawa, K. Ondrias, P. Nagy, S. B. King, J. E. Saavedra, L. K. Keefer, M. Singer, M. Kelm, A. R. Butler and M. Feelisch, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4651–4660 CrossRef PubMed.
- B. Fromenty, J. Hepatol., 2013, 58, 824–826 CrossRef PubMed.
- M. A. Robin, I. Sauvage, T. Grandperret, V. Descatoire, D. Pessayre and B. Fromenty, FEBS Lett., 2005, 579, 6895–6902 CrossRef CAS PubMed.
- S. Donthamsetty, V. S. Bhave, M. S. Mitra, J. R. Latendresse and H. M. Mehendale, Hepatology, 2007, 45, 391–403 CrossRef CAS PubMed.
- A. Michaut, C. Moreau, M. A. Robin and B. Fromenty, Liver Int., 2014, 34, 171–179 CrossRef PubMed.
- G. Tarantino, P. Conca, V. Basile, A. Gentile, D. Capone, G. Polichetti and E. Leo, Hepatol. Res., 2007, 37, 410–415 CrossRef CAS PubMed.
- D. Cheng, Y. Pan, L. Wang, Z. Zeng, L. Yuan, X. Zhang and Y. T. Chang, J. Am. Chem. Soc., 2017, 139, 285–292 CrossRef CAS PubMed.
- K. R. Olson and Y. Gao, Free Radical Biol. Med., 2019, 135, 1–14 CrossRef CAS PubMed.
- Y. Kimura, R. Dargusch, D. Schubert and H. Kimura, Antioxid. Redox Signaling, 2006, 8, 661–670 CrossRef CAS PubMed.
- Z. S. Xu, X. Y. Wang, D. M. Xiao, L. F. Hu, M. Lu, Z. Y. Wu and J. S. Bian, Free Radical Biol. Med., 2011, 50, 1314–1323 CrossRef CAS PubMed.
- A. E. Wagner, P. Huebbe, T. Konishi, M. M. Rahman, M. Nakahara, S. Matsugo and G. Rimbach, J. Agric. Food Chem., 2008, 56, 11694–11699 CrossRef CAS PubMed.
- P. Manna, N. Gungor, R. McVie and S. K. Jain, J. Biol. Chem., 2014, 289, 11767–11778 CrossRef CAS PubMed.
- Y. Pan, S. Ye, D. Yuan, J. Zhang, Y. Bai and C. Shao, Mutat. Res., 2012, 738–739, 12–18 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc03336g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: