Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phenol-like group functionalized graphene quantum dots structurally mimicking natural antioxidants for highly efficient acute kidney injury treatment†
Huan
Wang
ab,
Dongqin
Yu
ab,
Jiao
Fang
c,
Ya
Zhou
ab,
Daowei
Li
d,
Zhen
Liu
e,
Jinsong
Ren
 *ab and
Xiaogang
Qu
*ab and
Xiaogang
Qu
 *ab
*ab
aState Key Laboratory of Rare Earth Resources Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: jren@ciac.ac.cn; xqu@ciac.ac.cn
bUniversity of Science and Technology of China, Hefei 230029, P. R. China
cDepartment of Oral Implantology, School and Hospital of Stomatology, Jilin University, Changchun 130021, P. R. China
dJilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, School of Stomatology, Jilin University, Changchun 130021, P. R. China
eBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China
First published on 5th August 2020
Abstract
Acute kidney injury (AKI) is a syndrome characterized by rapid loss of renal excretory function with high in-hospital mortality. The excess generation of reactive oxygen species (ROS) in the kidneys during AKI has been considered a major cause of renal failure. Currently available antioxidants for AKI treatment often lack the required antioxidative efficacy or renal accumulation rate. Herein, inspired by the structure of natural phenolic antioxidants, phenol-like group functionalized graphene quantum dots (h-GQDs) with both high ROS scavenging efficacy and renal specificity are constructed for AKI antioxidative therapy. Similar to natural polyphenols, the abundant phenol-like groups on h-GQDs are demonstrated to be the active components exerting antioxidative effects. Further exhaustive mechanistic investigations indicate that the ultrahigh antioxidative activity of h-GQDs originates not solely from the phenol-like groups, but also from the synergy between adjacent phenol-like groups, as well as the removal of unfavorable carbonyl groups on h-GQDs. In AKI mice, h-GQDs can effectively protect the kidneys from oxidative injury with only a one-sixteenth dose of the clinical antioxidant N-acetylcysteine (NAC) and show no evidence of toxicity. The findings of this study will facilitate development of high-performance carbon-based antioxidative platforms via structure–activity relationships for treating AKI and other ROS-related diseases.
Introduction
Acute kidney injury (AKI) is a syndrome characterized by the rapid loss of excretory function of the kidneys and is associated with increasing risk of in-hospital death.1,2 Although significant achievements have been made to understand the pathogenesis of AKI, no specific therapies have emerged for effective AKI amelioration or expedited recovery. In current clinical settings, only renal replacement therapies (RRTs) are available for AKI amelioration; however, comprehensive kidney support is difficult to achieve.3,4 As a result, patients with AKI who are treated with RRT still have a high mortality rate of 50–60%, and nearly 20% of the surviving patients remain on dialysis after hospital discharge. During AKI, the reactive oxygen species (ROS) overproduction has been counted as a major cause of renal failure.5,6 Towards this goal, antioxidative therapy via efficient ROS scavenging is regarded as one of the critical approaches to protect renal function in AKI. Although small molecular antioxidants have shown positive effects in alleviating contrast agent-induced renal damage, such as N-acetylcysteine (NAC), their poor bio-availability and specificity hinder the clinical availability to other types of AKI.5,7Alternatively, ultrasmall nanomaterials with both renal specificity and antioxidative activity have emerged as potential candidates for alleviating AKI.6–8 Compared with small molecular drugs, these ultrasmall nanoagents with reasonable sizes can remain in the kidneys for a significantly longer period and have better targeting ability.9,10 For instance, antioxidative nanoclusters based on the variable valence state of metal centers have been developed for AKI treatment.6 However, these metal-containing nanoagents usually pose some safety concerns associated with their unwanted metal ion leakage and possible residues.11 Additionally, metal-free nanoagents such as DNA origami nanostructures and black phosphorus nanosheets have been used to alleviate AKI.7,12 However, translation of these nanoagents faces several potential roadblocks including ambiguous in vivo stability, indefinite immune responses, and potential acute toxicity.13,14 As of now, the engineering of antioxidative nanoagents for treating renal diseases is still in its infancy.15,16 Hence, it remains challenging to design novel nanoplatforms for AKI treatment with great antioxidative activity, high renal accumulation, and high biocompatibility.
Owing to their high stability, low toxicity, and facile production on a large scale, nanocarbons have arisen as favorable candidates for biomedical applications.17 Recently, a series of carbogenic nanodots, such as graphene quantum dots (GQDs), nitrogen-doped carbon dots, selenium-doped carbon dots, chlorine-doped GQDs, and nitrogen/sulphur-co-doped carbon dots, have emerged as antioxidants for ROS elimination.18–25 In particular, GQDs have shown efficient renal clearance properties and long body retention, providing them great kidney targeting ability.26,27 Significantly, the biological effects of GQDs functionalized with enriched oxygenated groups have been investigated exhaustively, and they exhibit high biocompatibility both in vitro and in vivo even at a high dose.28,29 However, such GQDs usually have relatively low antioxidative activity, which greatly limits their wide biological usage under various physiological and pathological conditions, let alone developing the antioxidative effect of GQDs for treating AKI. More importantly, the design of high-efficacy GQD-based antioxidative platforms via engineering surface oxygenated groups has not been well explored.21
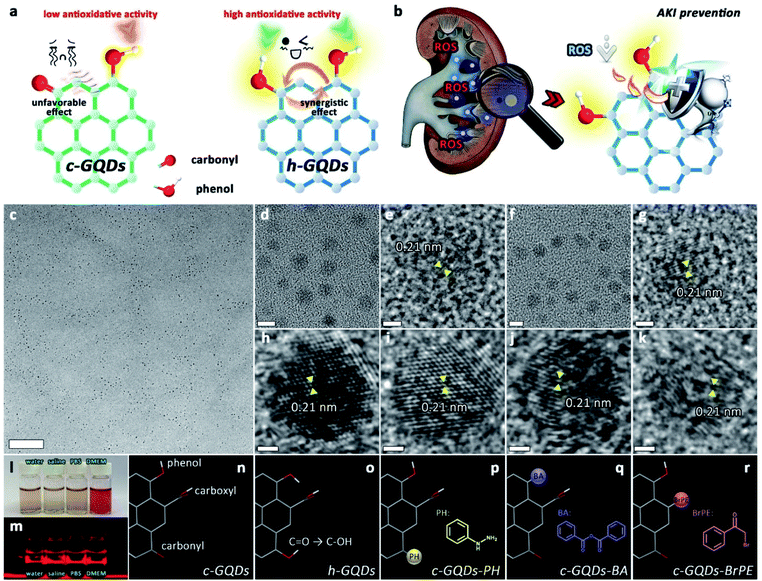
As important natural antioxidants, polyphenols show pronounced biological effects including high physiological and pharmacological activities. Generally, the phenolic groups of polyphenolic antioxidants act as H-atom donors for ROS trapping.30,31 A phenolic H-atom is captured by a free radical, leading to the formation of an intermediate phenoxyl radical. The phenoxyl radical so formed is resonance-stabilized by p–π orbital overlap until it encounters a further free radical with which it couples rapidly.30 Additionally, the antioxidative activity of polyphenols could be greatly influenced by various substituent groups. Generally, there are various oxygenated groups, such as hydroxyl (C–OH), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and carboxyl (COOH), on GQDs. The C–OH species can attach either to the sp2 carbon centers or the sp3 carbon centers, and the sp2 carbon center-bonded C–OH species on the graphene structure are termed “phenol-like groups”.32–35 Inspired by these principles, we envision that the phenol-like groups on the graphene structure can act as H-atom donors for ROS trapping, and the related intermediate radicals could be well stabilized by the long-range π-electron system. Meanwhile, other surface oxygenated groups may have positive or negative effects on the antioxidative activity. Herein, we present phenol-like group functionalized graphene quantum dots (h-GQDs) with preferential renal accumulation as high-efficacy ROS scavengers for treating AKI (Fig. 1b). As expected, the phenol-like functionalities on h-GQDs were demonstrated to be the active components for the antioxidative activity. Further exhaustive mechanistic investigations indicate that the ROS scavenging capacity of the phenol-like groups on the graphene structure can be greatly suppressed by nearby carbonyl groups and significantly enhanced by other adjacent phenol-like moieties (Fig. 1a). Therefore, the observed high antioxidative activity of h-GQDs originates from the well-tailored specific oxygenated groups including increased amounts of phenol-like groups and decreased amounts of carbonyl groups. Benefitting from their reasonable sizes, these h-GQDs show high renal accumulation in AKI mice over a long period of time (>72 h). As a result, these h-GQDs can exert comparable therapeutic effects with only a one-sixteenth dose of the clinical antioxidant N-acetylcysteine (NAC) and show no evidence of toxicity. We expect that this study could facilitate the development of high-efficacy carbon-based antioxidative platforms via structure–activity relationships to address the practical needs of treating AKI and other ROS-related diseases.
O), and carboxyl (COOH), on GQDs. The C–OH species can attach either to the sp2 carbon centers or the sp3 carbon centers, and the sp2 carbon center-bonded C–OH species on the graphene structure are termed “phenol-like groups”.32–35 Inspired by these principles, we envision that the phenol-like groups on the graphene structure can act as H-atom donors for ROS trapping, and the related intermediate radicals could be well stabilized by the long-range π-electron system. Meanwhile, other surface oxygenated groups may have positive or negative effects on the antioxidative activity. Herein, we present phenol-like group functionalized graphene quantum dots (h-GQDs) with preferential renal accumulation as high-efficacy ROS scavengers for treating AKI (Fig. 1b). As expected, the phenol-like functionalities on h-GQDs were demonstrated to be the active components for the antioxidative activity. Further exhaustive mechanistic investigations indicate that the ROS scavenging capacity of the phenol-like groups on the graphene structure can be greatly suppressed by nearby carbonyl groups and significantly enhanced by other adjacent phenol-like moieties (Fig. 1a). Therefore, the observed high antioxidative activity of h-GQDs originates from the well-tailored specific oxygenated groups including increased amounts of phenol-like groups and decreased amounts of carbonyl groups. Benefitting from their reasonable sizes, these h-GQDs show high renal accumulation in AKI mice over a long period of time (>72 h). As a result, these h-GQDs can exert comparable therapeutic effects with only a one-sixteenth dose of the clinical antioxidant N-acetylcysteine (NAC) and show no evidence of toxicity. We expect that this study could facilitate the development of high-efficacy carbon-based antioxidative platforms via structure–activity relationships to address the practical needs of treating AKI and other ROS-related diseases.
Results and discussion
Classical GQDs (c-GQDs) with enriched oxygenated groups including C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and COOH were first prepared as precursors.36 As an efficient reductant, NaBH4 could selectively reduce C
O, C–OH, and COOH were first prepared as precursors.36 As an efficient reductant, NaBH4 could selectively reduce C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to C–OH and significantly increase the amount of C–OH on carbon without reducing other species.37–39 In our present design, C
O to C–OH and significantly increase the amount of C–OH on carbon without reducing other species.37–39 In our present design, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on c-GQDs was efficiently converted to C–OH to result in phenol-like group functionalized graphene quantum dots (h-GQDs) by using an appropriate amount of NaBH4 as the reductant. For exploration of the detailed roles of various oxygenated groups in the antioxidative activity, phenylhydrazine (PH), benzoic anhydride (BA), and 2-bromo-1-phenylethanone (BrPE) were employed as high-specificity deactivating agents to react with C
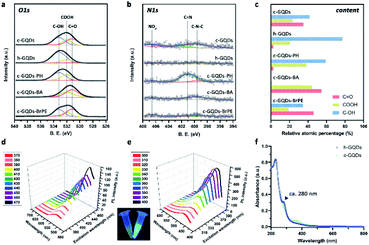
O on c-GQDs was efficiently converted to C–OH to result in phenol-like group functionalized graphene quantum dots (h-GQDs) by using an appropriate amount of NaBH4 as the reductant. For exploration of the detailed roles of various oxygenated groups in the antioxidative activity, phenylhydrazine (PH), benzoic anhydride (BA), and 2-bromo-1-phenylethanone (BrPE) were employed as high-specificity deactivating agents to react with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and COOH on c-GQDs, which were defined as c-GQDs–PH, c-GQDs–BA, and c-GQDs–BrPE, respectively (Fig. S1†).36,40 As shown in Fig. 1c–k, transmission electron microscope (TEM) images demonstrated that there were negligible differences in size and morphology among various GQDs. All these GQDs were extremely homogeneous with an average size of 4.4–4.8 nm (Fig. S2†). High-resolution TEM (HR-TEM) images indicated that all the GQDs had fine crystallinity with a lattice spacing of 0.21 nm, which could be attributed to the characteristic (102) diffraction planes of the sp2 graphitic carbon (Fig. 1e, g, h–k). High-resolution O1s X-ray photoelectron spectroscopy (XPS) was used to explore the distribution of different oxygenated groups on various GQDs in detail. As shown in Fig. S3a,† the total oxygen content of h-GQDs decreased only slightly after reduction. As shown in Fig. 2a, pristine c-GQDs were mainly covered with C
O, C–OH, and COOH on c-GQDs, which were defined as c-GQDs–PH, c-GQDs–BA, and c-GQDs–BrPE, respectively (Fig. S1†).36,40 As shown in Fig. 1c–k, transmission electron microscope (TEM) images demonstrated that there were negligible differences in size and morphology among various GQDs. All these GQDs were extremely homogeneous with an average size of 4.4–4.8 nm (Fig. S2†). High-resolution TEM (HR-TEM) images indicated that all the GQDs had fine crystallinity with a lattice spacing of 0.21 nm, which could be attributed to the characteristic (102) diffraction planes of the sp2 graphitic carbon (Fig. 1e, g, h–k). High-resolution O1s X-ray photoelectron spectroscopy (XPS) was used to explore the distribution of different oxygenated groups on various GQDs in detail. As shown in Fig. S3a,† the total oxygen content of h-GQDs decreased only slightly after reduction. As shown in Fig. 2a, pristine c-GQDs were mainly covered with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and COOH species. After reduction, both the dramatic decrease in intensity of the C
O, C–OH, and COOH species. After reduction, both the dramatic decrease in intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal and the significant increase in intensity of the C–OH signal could be observed, and only some COOH was removed. Notably, the content of C–OH accounted for nearly three-quarters of all oxygenated groups on h-GQDs (Fig. 2c). These results demonstrated that the C
O signal and the significant increase in intensity of the C–OH signal could be observed, and only some COOH was removed. Notably, the content of C–OH accounted for nearly three-quarters of all oxygenated groups on h-GQDs (Fig. 2c). These results demonstrated that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was efficiently converted to C–OH during the reduction process. Fourier transform infrared spectroscopy (FT-IR) was further carried out to confirm the formation of new functional groups on h-GQDs. Compared with c-GQDs, h-GQDs showed a significant decrease of the C
O was efficiently converted to C–OH during the reduction process. Fourier transform infrared spectroscopy (FT-IR) was further carried out to confirm the formation of new functional groups on h-GQDs. Compared with c-GQDs, h-GQDs showed a significant decrease of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal at 1710 cm−1 and an obvious increase of the C–OH signal at 3415 cm−1 (Fig. S3b†). Importantly, a new vibrational band at 1200 cm−1 could be detected and could be assigned to the typical C–O stretching of phenolic moieties on the graphene structure.33 Such results indicated that the abundant newly formed C–OH species on h-GQDs contained phenol-like moieties. Then, the optical properties of c-GQDs and h-GQDs were investigated to reveal their surface chemistry. In the UV-Vis-NIR absorption spectra, the absorption peak at ca. 370 nm of c-GQDs disappeared after NaBH4 reduction, and a new absorption peak at ca. 280 nm appeared (Fig. 2f).38 In the photoluminescence (PL) spectra, the c-GQDs showed PL excitation (PLE) and PL peaks at 470 and 515 nm, respectively (Fig. 2d and S4a†). As for h-GQDs, blue-shifted PLE and PL peaks at 400 and 465 nm were observed (Fig. 2e and S4b†). It has been known that the phenol-like functionalities on GQDs contributed the most to the blue emissions and the luminescence enhancement, whereas the carbonyl groups on GQDs contributed to the green emissions.34,35,38,41,42 These results collectively demonstrated the removal of carbonyl groups and the functionalization of phenol-like groups on c-GQDs during the reduction process. Then, XPS analysis was used to verify the occurrence of the deactivation process of various oxygenated groups on c-GQDs. Compared with c-GQDs, the decrease in the intensities of the C–OH signal for c-GQDs–BA and C
O signal at 1710 cm−1 and an obvious increase of the C–OH signal at 3415 cm−1 (Fig. S3b†). Importantly, a new vibrational band at 1200 cm−1 could be detected and could be assigned to the typical C–O stretching of phenolic moieties on the graphene structure.33 Such results indicated that the abundant newly formed C–OH species on h-GQDs contained phenol-like moieties. Then, the optical properties of c-GQDs and h-GQDs were investigated to reveal their surface chemistry. In the UV-Vis-NIR absorption spectra, the absorption peak at ca. 370 nm of c-GQDs disappeared after NaBH4 reduction, and a new absorption peak at ca. 280 nm appeared (Fig. 2f).38 In the photoluminescence (PL) spectra, the c-GQDs showed PL excitation (PLE) and PL peaks at 470 and 515 nm, respectively (Fig. 2d and S4a†). As for h-GQDs, blue-shifted PLE and PL peaks at 400 and 465 nm were observed (Fig. 2e and S4b†). It has been known that the phenol-like functionalities on GQDs contributed the most to the blue emissions and the luminescence enhancement, whereas the carbonyl groups on GQDs contributed to the green emissions.34,35,38,41,42 These results collectively demonstrated the removal of carbonyl groups and the functionalization of phenol-like groups on c-GQDs during the reduction process. Then, XPS analysis was used to verify the occurrence of the deactivation process of various oxygenated groups on c-GQDs. Compared with c-GQDs, the decrease in the intensities of the C–OH signal for c-GQDs–BA and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal for c-GQDs–PH and the increase in the C
O signal for c-GQDs–PH and the increase in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal for c-GQDs–BrPE could be clearly detected in the O1s XPS spectra, indicating that the three reactions had indeed occurred (Fig. 2a).36 Additionally, the presence of the N1s peak in c-GQDs–PH could be ascribed to the formation of C
O signal for c-GQDs–BrPE could be clearly detected in the O1s XPS spectra, indicating that the three reactions had indeed occurred (Fig. 2a).36 Additionally, the presence of the N1s peak in c-GQDs–PH could be ascribed to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds (Fig. 2b). We further identified the ζ potentials of various GQDs. As shown in Fig. S5,† the ζ potential value of h-GQDs was higher than that of c-GQDs. Considering that there was no obvious change in the total oxygen content of h-GQDs after reduction, the increased ζ potential value of h-GQDs could be attributed to the removal of negatively charged C
N bonds (Fig. 2b). We further identified the ζ potentials of various GQDs. As shown in Fig. S5,† the ζ potential value of h-GQDs was higher than that of c-GQDs. Considering that there was no obvious change in the total oxygen content of h-GQDs after reduction, the increased ζ potential value of h-GQDs could be attributed to the removal of negatively charged C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Moreover, the removal of COOH from c-GQDs by BrPE could lead to the formation c-GQDs–BrPE with a higher ζ potential value. The chemical structures of various GQDs are illustrated in Fig. 1n–r. All these results demonstrated the successful construction of phenol-like group functionalized h-GQDs and the occurrence of the deactivation process of various oxygenated groups on c-GQDs. Benefitting from the enriched oxygenated groups, all these GQDs showed excellent solubility in water and physiological solutions, and distinct Tyndall effects could be well observed (Fig. 1l and m, and S6†).
O. Moreover, the removal of COOH from c-GQDs by BrPE could lead to the formation c-GQDs–BrPE with a higher ζ potential value. The chemical structures of various GQDs are illustrated in Fig. 1n–r. All these results demonstrated the successful construction of phenol-like group functionalized h-GQDs and the occurrence of the deactivation process of various oxygenated groups on c-GQDs. Benefitting from the enriched oxygenated groups, all these GQDs showed excellent solubility in water and physiological solutions, and distinct Tyndall effects could be well observed (Fig. 1l and m, and S6†).
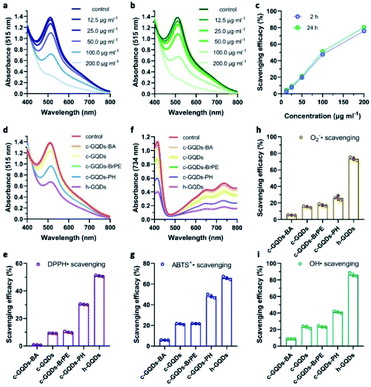
The DPPH˙ assay was used to investigate the H-atom donating ability of h-GQDs for antioxidative activity. The change of absorbance of DPPH˙ at 515 nm was monitored after co-incubating with h-GQDs for 2 h. As shown in Fig. 3a, these h-GQDs exhibited extremely high antioxidative activity in a concentration-dependent manner. As shown in Fig. 3b and c, the DPPH˙ scavenging efficacies did not show obvious changes when the co-incubation time was extended to 24 h, suggesting that most of the labile H-atoms on h-GQDs were exhausted within 2 h of reaction time. Compared with c-GQDs, these h-GQDs exhibited at least 5 times higher activity under the same experimental conditions (Fig. 3d and e). We further explored the DPPH˙ scavenging efficacies of various GQDs with deactivated oxygenated groups to understand the contribution of different oxygenated groups toward the activity. Compared with c-GQDs, the activity of c-GQDs–BA dropped by about 90%, indicating that the antioxidative activity of c-GQDs comes mainly from C–OH species (Fig. 3d and e). Moreover, the activity of c-GQDs–BrPE was similar to that of c-GQDs. This is because the carboxyl groups and nonphenolic hydroxyl groups are usually not able to serve as H-atom donors like phenolic groups for ROS trapping, which also suggests that the phenol-like groups on h-GQDs are the active components exerting antioxidative effects. Significantly, c-GQDs–PH exhibited obviously higher activity than c-GQDs, demonstrating that the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O could have unfavorable effects on the activity. Such results indicated that the observed high antioxidative activity of h-GQDs should not originate solely from the phenol-like moieties.
O could have unfavorable effects on the activity. Such results indicated that the observed high antioxidative activity of h-GQDs should not originate solely from the phenol-like moieties.
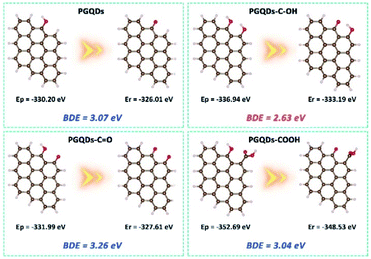
For natural phenolic antioxidants, the antioxidative activity could be greatly influenced by various ring substituents.30 To explore the real origin of the observed high activity of h-GQDs, the O–H bond dissociation energy (BDE) of the phenol-like group on the graphene structure (denoted as PGQDs) was studied by DFT calculations (Fig. 4). Generally, a relatively low O–H BDE of the phenolic moiety facilitates H-atom donation, suggesting a high antioxidative activity. The possible effects of various oxygenated groups on the activity of the phenol-like group on PGQDs were investigated by adding one specific oxygenated group (carbonyl, carboxyl, or phenol-like group) around the phenol-like group, which were denoted as PGQDs–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PGQDs–COOH, and PGQDs–C–OH, respectively. The BDE of the phenol-like group on PGQDs and PGQDs–COOH could be calculated to be 3.07 eV and 3.04 eV, whereas the BDE for PGQDs–C
O, PGQDs–COOH, and PGQDs–C–OH, respectively. The BDE of the phenol-like group on PGQDs and PGQDs–COOH could be calculated to be 3.07 eV and 3.04 eV, whereas the BDE for PGQDs–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was 3.26 eV. According to these results, the H donating ability of the phenol-like group on PGQDs could be greatly suppressed by the nearby carbonyl group. In addition, the approaching of the carboxyl group to the phenol-like group does not much affect the H-atom donating ability, consistent with our experimental results. Significantly, PGQDs–C–OH had the lowest BDE value of 2.63 eV, indicating that the energy barrier for O–H bond dissociation of the phenol-like group on PGQDs could be effectively reduced by other adjacent phenol-like groups.
O was 3.26 eV. According to these results, the H donating ability of the phenol-like group on PGQDs could be greatly suppressed by the nearby carbonyl group. In addition, the approaching of the carboxyl group to the phenol-like group does not much affect the H-atom donating ability, consistent with our experimental results. Significantly, PGQDs–C–OH had the lowest BDE value of 2.63 eV, indicating that the energy barrier for O–H bond dissociation of the phenol-like group on PGQDs could be effectively reduced by other adjacent phenol-like groups.
Comprehensive insight into the antioxidative activity of h-GQDs and the related mechanisms could be summarized as follows. As the active sites, the phenol-like groups could not only serve as H-atom donors to exert antioxidative effects, but also synergistically enhance the H-atom donating ability of other adjacent phenol-like groups and thus the antioxidative activity. However, the existence of carbonyl groups could decrease the H-atom donating ability of nearby phenol-like groups, causing unfavorable effects on the activity. Hence, h-GQDs with abundant phenol-like groups and negligible carbonyl groups exhibited extremely high antioxidative activity. Furthermore, we performed assays with ABTS+˙, superoxide radicals (˙O2−), and hydroxyl radicals (˙OH) to re-confirm the antioxidative properties of h-GQDs. As shown in Fig. 3f–i, h-GQDs could effectively eliminate ABTS+˙, ˙O2− and ˙OH under physiological conditions.43
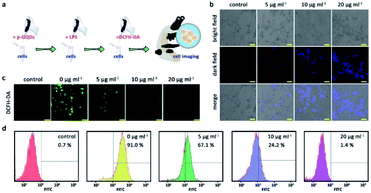
Considering their bio-related usages, h-GQDs were then non-covalently functionalized with PEG molecules (molecular weight: 1000) via the hydrophobic interaction between h-GQDs and terminal phospholipid groups of mPEG–DSPE, which were termed p-GQDs.44 Ultrasmall nanomaterials functionalized with PEG with a suitable molecular weight can retain their efficient renal clearance properties and provide a relatively longer body retention.45,46ζ potential measurements of p-GQDs indicated successful PEGylation, which was re-confirmed by FT-IR analysis (Fig. S3d and S5†). Moreover, h-GQDs and p-GQDs showed similar UV-Vis-NIR absorption spectra, indicating that the h-GQD cores were well preserved by PEGylation (Fig. S3c†). As shown in Fig. 1f and g, and S2c,† TEM images and size distribution analysis indicated that there were negligible differences in size and morphology between h-GQDs and p-GQDs. Significantly, the non-covalent PEGylation showed negligible influence on the antioxidative activity of h-GQDs (Fig. S7†). We then explored the antioxidative activity of p-GQDs in vitro by using HEK-293T cells, a typical human embryonic kidney cell line (Fig. 5a). The cytotoxicity of p-GQDs was evaluated at first. As shown in Fig. S8,† the MTT assay revealed that all the cell viabilities were not hindered by p-GQDs even at the highest concentration of 200 μg mL−1 after 24 h of co-incubation. Cellular uptake of p-GQDs could be visualized by fluorescence imaging. As expected, p-GQDs could be efficiently taken up by HEK-293T cells after 2 h of co-incubation in a concentration-dependent manner (Fig. 5b). Afterwards, LPS was used to stimulate cells to produce excess intracellular ROS, which could be evaluated by using DCFH-DA as a typical fluorescence indicator. As shown in Fig. 5c, cells treated with LPS revealed increased intracellular ROS levels. Upon the treatment of p-GQDs, it was clearly found that p-GQDs could effectively alleviate oxidative stress in vitro in a concentration-dependent manner, which was also re-confirmed by flow cytometry analysis (Fig. 5d).
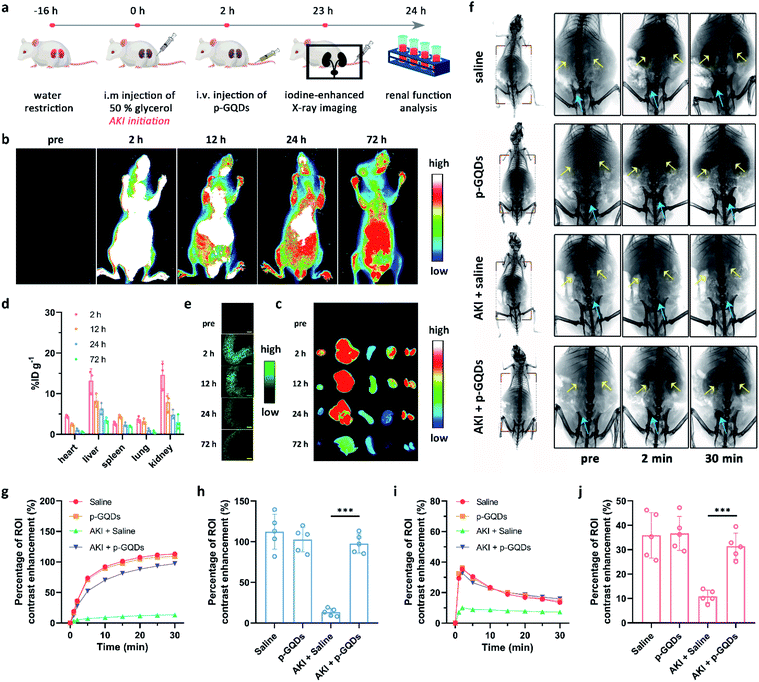
We then explored the bio-distribution of p-GQDs in AKI mice. A murine model of rhabdomyolysis-induced AKI was established via intramuscular (i.m.) injection of glycerol into dehydrated healthy mice at first (Fig. 6a).7 This time point was defined as the initiation of AKI. Time-dependent in vivo and ex vivo fluorescence imaging was carried out to monitor the bio-distribution of p-GQDs visually. The excitation wavelength of h-GQDs was around 400 nm with low penetration capacity, which was not suitable for bio-imaging studies. Thus, h-GQDs were non-covalently functionalized with DSPE–PEG-Cy5.5 to result in fluorescent p-GQDs (Cy5.5-p-GQDs).44 The successful construction of Cy5.5-p-GQDs was confirmed via PL spectra (Fig. S9†). 2 h after initiation of the AKI model, Cy5.5-p-GQDs were intravenously injected into the mice. Cy5.5-p-GQDs could be easily found in injured kidneys after intravenous (i.v.) injection in AKI mice (Fig. 6b and c). Significantly, fluorescence signals from Cy5.5-p-GQDs could also be detected in the kidneys even at 72 h post-injection, suggesting their long renal retention. We further explored the bio-distribution of Cy5.5-p-GQDs via the quantitative analysis of fluorescence signals achieved from the tissue homogenates ex vivo.47,48 As shown in Fig. 6d, most of these Cy5.5-p-GQDs still remained in the kidneys at 72 h post-injection, which could be ascribed to the efficient renal accumulation of these p-GQDs. Fig. 6e additionally describes the time-dependent accumulation of Cy5.5-p-GQDs in the kidneys by using confocal images of cryosections harvested from AKI mice after i.v. injection. All these results strongly evidenced the efficient renal accumulation properties and relatively long renal retention of p-GQDs in AKI mice and their great potential for further in vivo AKI treatment.
We then sought to use p-GQDs to alleviate AKI in vivo. Mice were randomly divided into four groups, which were defined as healthy mice + saline, healthy mice + p-GQDs (10 mg kg−1), AKI mice + saline, and AKI mice + p-GQDs (10 mg kg−1). Afterwards, mouse renal function was evaluated by iopromide-enhanced X-ray imaging at one day post-initiation of the AKI model.10,49Fig. 6f demonstrates that contrast enhancement of the kidneys in the groups of healthy mice + saline and healthy mice + p-GQDs reached the maximum peak within 2 min post-injection of iopromide and decreased dramatically over time, and the gradual bladder accumulation of iopromide was also clearly observed. The above results not only indicated that p-GQDs exhibited excellent safety, but also provided standard data for the following assessment of antioxidative therapy. In the group of AKI mice + saline, significantly decreased accumulation of iopromide was found in the kidneys with little excretion of iopromide to the bladder. By contrast, AKI mice treated with p-GQDs exhibited obviously recovered renal functions, and all the dynamic curves of iopromide in the kidneys and bladder were similar to those of healthy mice treated with saline or p-GQDs (Fig. 6g–j). These results indicated that p-GQDs could serve as efficient nanoagents to recover renal function in AKI mice.
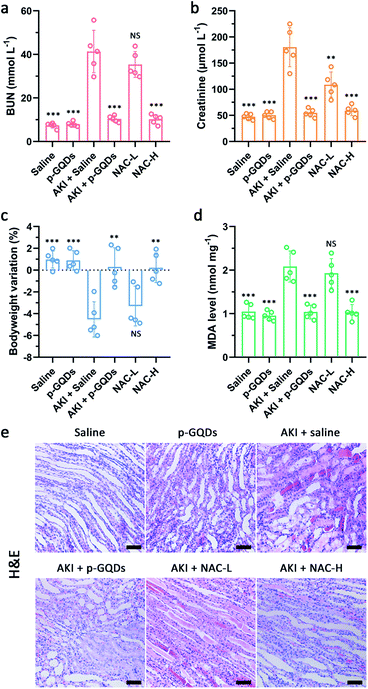
To verify the practical therapeutic efficacy of p-GQDs for treating AKI, NAC and PEGylated c-GQDs were selected and used as a positive control. In our experimental design, therapeutic efficacies of p-GQDs (10 mg kg−1), PEGylated c-GQDs (10 mg kg−1), high-dose NAC (NAC-H, 160 mg kg−1), and low-dose NAC (NAC-L, 10 mg kg−1) were investigated in detail. Generally, casts formed by denatured protein precipitation in renal tubules were utilized as characteristic indicators for kidney disease. As shown in Fig. 7e, S10e, and S11,† treatment effects based on hematoxylin and eosin (H&E) staining images indicated that damaged renal tubules and casts could be clearly observed in the kidney sections in the saline, NAC-L, and PEGylated c-GQD treatment groups while much fewer damaged structures were found in the p-GQD and NAC-H treatment groups, and nearly no damage was found within 7 days after p-GQD treatment. High levels of serum creatinine and blood urea nitrogen (BUN) were the clinical manifestations of the accumulation of end products of nitrogen metabolism, which could aid the evaluation of renal function in clinics. The levels of creatinine and BUN of AKI mice re-confirmed that p-GQDs and NAC-H could efficiently recover the renal function of AKI mice, while NAC-L and PEGylated c-GQDs did not produce obvious therapeutic effects (Fig. 7a and b, S10a and b, and S12a and b†). Moreover, the survival curve and body weight of AKI mice were also monitored, further suggesting that p-GQDs were highly efficient for AKI treatment (Fig. 7c, S10c, S13, and S14†). Generally, excess ROS generation could induce lipid peroxidation. Therefore, we further explored whether p-GQDs could relieve lipid peroxidation in the injured kidneys by using the malondialdehyde (MDA) assay. Indeed, lipid peroxidation of the injured kidneys in AKI mice could be relieved well by p-GQDs (Fig. 7d, S10d, and S12c†). Taken together, these results demonstrated the feasibility of p-GQDs as efficient antioxidants to protect kidneys from injury during AKI. Notably, benefitting from their high antioxidative activity, p-GQDs provided a much better therapeutic efficacy compared with PEGylated c-GQDs. Significantly, p-GQDs with a relatively low dose of 10 mg kg−1 had similar therapeutic effects to high-dose NAC (160 mg kg−1), further indicating the admirable therapeutic effect of p-GQDs even at a low dose.
Given their excellent efficacy for AKI treatment, we then explored the biocompatibility and systemic toxicity of p-GQDs in healthy mice. Compared with the control group, mice in the test group showed negligible differences in body weight, eating, drinking, and activity during the whole experimental period (Fig. S15†). H&E staining images of major organs including the heart, liver, spleen, lungs, and kidneys from mice 15 days after different treatments demonstrated that p-GQDs had high biocompatibility and negligible systemic toxicity (Fig. S16†). The results of hematology analysis and blood biochemical assay revealed that there were no significant differences between the test group and the control group, and all the parameters fell well within the reference index (Fig. S17–S20†).
Conclusions
In summary, phenol-like group functionalized graphene quantum dots (h-GQDs) were developed as high-efficacy ROS scavengers for treating AKI. These h-GQDs exhibited ultrahigh antioxidative activity and could scavenge multiple cytotoxic ROS. Mechanistically, the synergy between the functionalized phenol-like moieties as well as the removal of unfavorable carbonyl groups on h-GQDs collectively contributed to the observed high activity. The results of in vivo experiments demonstrated that the superb antioxidative activity, high renal accumulation, and excellent biocompatibility afforded these h-GQDs great potential for AKI treatment towards practical usage. We envision that this study could offer valuable insight into the design of high-performance carbon-based antioxidative platforms via structure–activity relationships for treating AKI and other ROS-related diseases.Ethical statement
All animal experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev. 1985) and approved by the Jilin University Animal Care and Use Committee. Balb/c mice (8–10 weeks, 25 g) were obtained from the Laboratory Animal Center of Jilin University (Changchun, China), and all animal care and handling procedures were in accordance with the guidelines approved by the ethics committee of Jilin University.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This study was supported by the Natural Science Foundation of China (21820102009, 21871249, 21533008, and 91856205) and the Key Program of Frontier of Sciences (CASQYZDJ-SSW-SLH052).Notes and references
- R. Bellomo, J. A. Kellum and C. Ronco, Acute kidney injury, Lancet, 2012, 380(9843), 756–766 CrossRef.
- R. Murugan and J. A. Kellum, Acute kidney injury: what's the prognosis?, Nat. Rev. Nephrol., 2011, 7, 209 CrossRef CAS PubMed.
- J. A. Kellum, R. Bellomo and C. Ronco, Progress in Prevention and Treatment of Acute Kidney Injury: Moving Beyond Kidney Attack, J. Am. Med. Assoc., 2018, 320(5), 437–438 CrossRef PubMed.
- E. A. J. Hoste and M. Schurgers, Epidemiology of acute kidney injury: How big is the problem?, Crit. Care Med., 2008, 36(4), S146–S151 CrossRef PubMed.
- M. Tepel, M. van der Giet, C. Schwarzfeld, U. Laufer, D. Liermann and W. Zidek, Prevention of Radiographic-Contrast-Agent-Induced Reductions in Renal Function by Acetylcysteine, N. Engl. J. Med., 2000, 343(3), 180–184 CrossRef CAS PubMed.
- D. Ni, D. Jiang, C. J. Kutyreff, J. Lai, Y. Yan, T. E. Barnhart, B. Yu, H.-J. Im, L. Kang, S. Y. Cho, Z. Liu, P. Huang, J. W. Engle and W. Cai, Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice, Nat. Commun., 2018, 9(1), 5421 CrossRef CAS PubMed.
- D. Jiang, Z. Ge, H.-J. Im, C. G. England, D. Ni, J. Hou, L. Zhang, C. J. Kutyreff, Y. Yan, Y. Liu, S. Y. Cho, J. W. Engle, J. Shi, P. Huang, C. Fan, H. Yan and W. Cai, DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury, Nat. Biomed. Eng., 2018, 2(11), 865–877 CrossRef CAS PubMed.
- Z. Rosenkrans, T. Sun, D. Jiang, C. Ferreira, W. Chen, D. Ni, J. Engle and W. Cai, Renal-Accumulating Selenium-Doped Carbon Quantum Dots Treat Acute Kidney Injury in Mice, J. Nucl. Med., 2019, 60(suppl. 1), 411 Search PubMed.
- H. Soo Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Renal clearance of quantum dots, Nat. Biotechnol., 2007, 25, 1165 CrossRef PubMed.
- B. Du, X. Jiang, A. Das, Q. Zhou, M. Yu, R. Jin and J. Zheng, Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime, Nat. Nanotechnol., 2017, 12, 1096 CrossRef CAS PubMed.
- L. R. Arrington and G. K. Davis, Molybdenum Toxicity in the Rabbit: Two Figures, J. Nutr., 1953, 51(2), 295–304 CrossRef CAS PubMed.
- J. Hou, H. Wang, Z. Ge, T. Zuo, Q. Chen, X. Liu, S. Mou, C. Fan, Y. Xie and L. Wang, Treating Acute Kidney Injury with Antioxidative Black Phosphorus Nanosheets, Nano Lett., 2020, 20, 1447–1454 CrossRef CAS PubMed.
- S. Surana, A. R. Shenoy and Y. Krishnan, Designing DNA nanodevices for compatibility with the immune system of higher organisms, Nat. Nanotechnol., 2015, 10, 741 CrossRef CAS PubMed.
- X. Mu, J.-Y. Wang, X. Bai, F. Xu, H. Liu, J. Yang, Y. Jing, L. Liu, X. Xue, H. Dai, Q. Liu, Y.-M. Sun, C. Liu and X.-D. Zhang, Black Phosphorus Quantum Dot Induced Oxidative Stress and Toxicity in Living Cells and Mice, ACS Appl. Mater. Interfaces, 2017, 9, 20399–20409 CrossRef CAS PubMed.
- T. Yoshitomi, A. Hirayama and Y. Nagasaki, The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles, Biomaterials, 2011, 32(31), 8021–8028 CrossRef CAS PubMed.
- T. Sun, Z. Rosenkrans, D. Jiang, D. Ni, C. Ferreira, W. Chen, J. Engle and W. Cai, Zirconium-89 radiolabeled hollow ferulic acid nanostructure for PET imaging guided acute kidney injury therapy, J. Nucl. Med., 2019, 60(suppl. 1), 416 Search PubMed.
- S. Chung, R. A. Revia and M. Zhang, Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy, Adv. Mater., 2019, 1904362 CrossRef PubMed.
- Y. Chong, C. Ge, G. Fang, X. Tian, X. Ma, T. Wen, W. G. Wamer, C. Chen, Z. Chai and J.-J. Yin, Crossover between Anti- and Pro-oxidant Activities of Graphene Quantum Dots in the Absence or Presence of Light, ACS Nano, 2016, 10(9), 8690–8699 CrossRef CAS PubMed.
- E. L. G. Samuel, D. C. Marcano, V. Berka, B. R. Bitner, G. Wu, A. Potter, R. H. Fabian, R. G. Pautler, T. A. Kent, A.-L. Tsai and J. M. Tour, Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(8), 2343 CrossRef CAS PubMed.
- V. Ruiz, L. Yate, I. García, G. Cabanero and H.-J. Grande, Tuning the antioxidant activity of graphene quantum dots: Protective nanomaterials against dye decoloration, Carbon, 2017, 116, 366–374 CrossRef CAS.
- Y. Wang, W. Kong, L. Wang, J. Z. Zhang, Y. Li, X. Liu and Y. Li, Optimizing oxygen functional groups in graphene quantum dots for improved antioxidant mechanism, Phys. Chem. Chem. Phys., 2019, 21(3), 1336–1343 RSC.
- X. Mu, H. He, J. Wang, W. Long, Q. Li, H. Liu, Y. Gao, L. Ouyang, Q. Ren, S. Sun, J. Wang, J. Yang, Q. Liu, Y. Sun, C. Liu, X.-D. Zhang and W. Hu, Carbogenic Nanozyme with Ultrahigh Reactive Nitrogen Species Selectivity for Traumatic Brain Injury, Nano Lett., 2019, 19(7), 4527–4534 CrossRef CAS PubMed.
- F. Li, T. Li, C. Sun, J. Xia, Y. Jiao and H. Xu, Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging, Angew. Chem., Int. Ed., 2017, 56(33), 9910–9914 CrossRef CAS PubMed.
- L. Wang, Y. Li, Y. Wang, W. Kong, Q. Lu, X. Liu, D. Zhang and L. Qu, Chlorine-Doped Graphene Quantum Dots with Enhanced Anti- and Pro-Oxidant Properties, ACS Appl. Mater. Interfaces, 2019, 11(24), 21822–21829 CrossRef CAS PubMed.
- S. Zhao, M. Lan, X. Zhu, H. Xue, T.-W. Ng, X. Meng, C.-S. Lee, P. Wang and W. Zhang, Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging, ACS Appl. Mater. Interfaces, 2015, 7(31), 17054–17060 CrossRef CAS PubMed.
- H. Wang, R. Revia, K. Wang, R. J. Kant, Q. Mu, Z. Gai, K. Hong and M. Zhang, Paramagnetic Properties of Metal-Free Boron-Doped Graphene Quantum Dots and Their Application for Safe Magnetic Resonance Imaging, Adv. Mater., 2017, 29(11), 1605416 CrossRef PubMed.
- M. Nurunnabi, Z. Khatun, K. M. Huh, S. Y. Park, D. Y. Lee, K. J. Cho and Y.-k. Lee, In Vivo Biodistribution and Toxicology of Carboxylated Graphene Quantum Dots, ACS Nano, 2013, 7(8), 6858–6867 CrossRef CAS PubMed.
- Y. Chong, Y. Ma, H. Shen, X. Tu, X. Zhou, J. Xu, J. Dai, S. Fan and Z. Zhang, The in vitro and in vivo toxicity of graphene quantum dots, Biomaterials, 2014, 35(19), 5041–5048 CrossRef CAS PubMed.
- D. Zhang, Z. Zhang, Y. Wu, K. Fu, Y. Chen, W. Li and M. Chu, Systematic evaluation of graphene quantum dot toxicity to male mouse sexual behaviors, reproductive and offspring health, Biomaterials, 2019, 194, 215–232 CrossRef CAS PubMed.
- K. U. Ingold and D. A. Pratt, Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective, Chem. Rev., 2014, 114(18), 9022–9046 CrossRef CAS PubMed.
- Y. Liu and J. Shi, Antioxidative nanomaterials and biomedical applications, Nano Today, 2019, 27, 146–177 CrossRef CAS.
- E. Fuente, J. A. Menéndez, M. A. Díez, D. Suárez and M. A. Montes-Morán, Infrared Spectroscopy of Carbon Materials:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Quantum Chemical Study of Model Compounds, J. Phys. Chem. B, 2003, 107(26), 6350–6359 CrossRef CAS.
A Quantum Chemical Study of Model Compounds, J. Phys. Chem. B, 2003, 107(26), 6350–6359 CrossRef CAS. - T. Szabó, O. Berkesi, P. Forgó, K. Josepovits, Y. Sanakis, D. Petridis and I. Dékány, Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides, Chem. Mater., 2006, 18(11), 2740–2749 CrossRef.
- W. Zhang, Y. Liu, X. Meng, T. Ding, Y. Xu, H. Xu, Y. Ren, B. Liu, J. Huang, J. Yang and X. Fang, Graphenol defects induced blue emission enhancement in chemically reduced graphene quantum dots, Phys. Chem. Chem. Phys., 2015, 17(34), 22361–22366 RSC.
- W. Zhang, Y. Wang, X. Liu, X. Meng, H. Xu, Y. Xu, B. Liu, X. Fang, H.-B. Li and T. Ding, Insight into the multiple quasi-molecular states in ethylenediamine reduced graphene nanodots, Phys. Chem. Chem. Phys., 2017, 19(42), 28653–28665 RSC.
- H. Sun, A. Zhao, N. Gao, K. Li, J. Ren and X. Qu, Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots, Angew. Chem., Int. Ed., 2015, 54(24), 7176–7180 CrossRef CAS PubMed.
- H.-J. Shin, K. K. Kim, A. Benayad, S.-M. Yoon, H. K. Park, I.-S. Jung, M. H. Jin, H.-K. Jeong, J. M. Kim, J.-Y. Choi and Y. H. Lee, Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance, Adv. Funct. Mater., 2009, 19(12), 1987–1992 CrossRef CAS.
- H. Zheng, Q. Wang, Y. Long, H. Zhang, X. Huang and R. Zhu, Enhancing the luminescence of carbon dots with a reduction pathway, Chem. Commun., 2011, 47(38), 10650–10652 RSC.
- C. K. Chua and M. Pumera, Chemical reduction of graphene oxide: a synthetic chemistry viewpoint, Chem. Soc. Rev., 2014, 43(1), 291–312 RSC.
- W. Qi, W. Liu, B. Zhang, X. Gu, X. Guo and D. Su, Oxidative Dehydrogenation on Nanocarbon: Identification and Quantification of Active Sites by Chemical Titration, Angew. Chem., Int. Ed., 2013, 52(52), 14224–14228 CrossRef CAS PubMed.
- S. Zhu, J. Shao, Y. Song, X. Zhao, J. Du, L. Wang, H. Wang, K. Zhang, J. Zhang and B. Yang, Investigating the surface state of graphene quantum dots, Nanoscale, 2015, 7(17), 7927–7933 RSC.
- L. Wang, S.-J. Zhu, H.-Y. Wang, S.-N. Qu, Y.-L. Zhang, J.-H. Zhang, Q.-D. Chen, H.-L. Xu, W. Han, B. Yang and H.-B. Sun, Common Origin of Green Luminescence in Carbon Nanodots and Graphene Quantum Dots, ACS Nano, 2014, 8(3), 2541–2547 CrossRef CAS PubMed.
- H. Wang, D. Yu, B. Li, Z. Liu, J. Ren and X. Qu, Ultrasensitive magnetic resonance imaging of systemic reactive oxygen species in vivo for early diagnosis of sepsis using activatable nanoprobes, Chem. Sci., 2019, 10(13), 3770–3778 RSC.
- Z. Liu, C. Davis, W. Cai, L. He, X. Chen and H. Dai, Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(5), 1410 CrossRef CAS PubMed.
- L. Cheng, D. Jiang, A. Kamkaew, H. F. Valdovinos, H.-J. Im, L. Feng, C. G. England, S. Goel, T. E. Barnhart, Z. Liu and W. Cai, Renal-Clearable PEGylated Porphyrin Nanoparticles for Image-Guided Photodynamic Cancer Therapy, Adv. Funct. Mater., 2017, 27(34), 1702928 CrossRef PubMed.
- S. Shen, D. Jiang, L. Cheng, Y. Chao, K. Nie, Z. Dong, C. J. Kutyreff, J. W. Engle, P. Huang, W. Cai and Z. Liu, Renal-Clearable Ultrasmall Coordination Polymer Nanodots for Chelator-Free 64Cu-Labeling and Imaging-Guided Enhanced Radiotherapy of Cancer, ACS Nano, 2017, 11(9), 9103–9111 CrossRef CAS PubMed.
- H. Wan, J. Yue, S. Zhu, T. Uno, X. Zhang, Q. Yang, K. Yu, G. Hong, J. Wang, L. Li, Z. Ma, H. Gao, Y. Zhong, J. Su, A. L. Antaris, Y. Xia, J. Luo, Y. Liang and H. Dai, A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues, Nat. Commun., 2018, 9(1), 1171 CrossRef PubMed.
- R. Gao, X. Mei, D. Yan, R. Liang and M. Wei, Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy, Nat. Commun., 2018, 9(1), 2798 CrossRef PubMed.
- J. Xu, M. Yu, P. Carter, E. Hernandez, A. Dang, P. Kapur, J.-T. Hsieh and J. Zheng, In Vivo X-ray Imaging of Transport of Renal Clearable Gold Nanoparticles in the Kidneys, Angew. Chem., Int. Ed., 2017, 56(43), 13356–13360 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc03246h |
| This journal is © The Royal Society of Chemistry 2020 |