Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual-targeted photothermal agents for enhanced cancer therapy
Kaiye
Wang†
 ,
Yanan
Xiang†
,
Yanan
Xiang†
 ,
Wei
Pan
,
Wei
Pan
 ,
Hongyu
Wang
,
Hongyu
Wang
 *,
Na
Li
*,
Na
Li
 * and
Bo
Tang
* and
Bo
Tang
 *
*
College of Chemistry, Chemical Engineering and Materials Science, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Collaborative Innovation Centre of Functionalized Probes for Chemical Imaging in Universities of Shandong, Institute of Molecular and Nano Science, Shandong Normal University, Jinan, 250014, P. R. China. E-mail: lina@sdnu.edu.cn; tangb@sdnu.edu.cn
First published on 17th July 2020
Abstract
Photothermal therapy, in which light is converted into heat and triggers local hyperthermia to ablate tumors, presents an inherently specific and noninvasive treatment for tumor tissues. In this area, the development of efficient photothermal agents (PTAs) has always been a central topic. Although many efforts have been made on the investigation of novel molecular architectures and photothermal materials over the past decades, PTAs can cause severe damage to normal tissues because of the poor tumor aggregate ability and high irradiation density. Recently, dual-targeted photothermal agents (DTPTAs) provide an attractive strategy to overcome these problems and enhance cancer therapy. DTPTAs are functionalized with two classes of targeting units, including tumor environment targeting sites, tumor targeting sites and organelle targeting sites. In this perspective, typical targeted ligands and representative examples of photothermal therapeutic agents with dual-targeted properties are systematically summarized and recent advances using DTPTAs in tumor therapy are highlighted.
1. Introduction
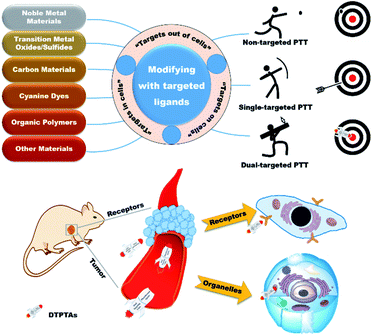
Cancer, as one of the most intractable diseases, has spoiled millions of lives due to its high morbidity and mortality.1,2 Over the past decades, great efforts have been focused on the development of efficient therapeutics for cancer treatment, such as surgery,3 radiotherapy,4,5 chemotherapy,6,7 photodynamic therapy (PDT),8–10 photothermal therapy (PTT)11–13 and immunotherapy.14–16 In particular, PTT has attracted extensive attention due to its inherent advantages of minimal invasion and external light control. According to the mechanism of PTT for cancer therapy, it achieves the goal of killing cancer cells via converting light energy to heat in order to trigger local hyperthermia in tumor, so PTT does not suffer from the limitations in hypoxic environments compared to PDT. Up to now, PTT as an important and promising treatment option has been a focus of attention to develop potential photothermal agents (PTAs) over the past decades. A broad range of novel inorganic and organic materials have been designed as PTAs to ablate tumor tissues. Although great advances have been made in the development of PTAs, it has been found that hyperpyrexia can seriously damage surrounding normal tissues because of poor tumor aggregation and high intensity irradiation. Hence, the development of efficacious PTAs with excellent tumor inhibition and negligible side effect presents a challenging task for cancer therapy.The rapid growth and proliferation of tumors result in abnormal demand and metabolism, which is beneficial for distinguishing normal tissues from diseased tissues. Therefore, targeted therapeutic agents exhibit enormously potential applications in PTT and have received extensive attention.17,18 Unfortunately, although single-targeted PTAs have made great achievements in cancer therapy, it is nonnegligible that off-targeted activities occur occasionally due to the dynamics and saturability of receptors on cancer cells.19,20 For further access to the tumor tissues, dual-targeted photothermal agents (DTPTAs) have been developed to ablate solid tumors, with slightly adverse effects (Scheme 1).21 Modified with two different targeted groups, DTPTAs show highly precise locating capability. Besides this, DTPTAs can trigger cell necrosis at low temperature and cause negligible damage to surrounding tissues when one of the targeted ligands tends to aggregate in the organelle, where heat-sensitive proteins and DNA are enriched.
In this perspective, typical targets are classified from three aspects based on the position of recognition sites, namely “extracellular targets”, “intracellular targets” and “subcellular targets” (Table 1). Furthermore, the applications of dual-targeted photothermal materials are carefully introduced. DTPTAs are described on the basis of different materials, including noble metal materials, transition metal oxide/sulfide materials, carbon materials, cyanine dyes, and organic polymers, as well as other materials. Photothermal conversion capability and tumor inhibition are also described in detail.
| Typical targets | Specific sites or stimulation | Targeted moieties |
|---|---|---|
| a Anti-CCL-28: a ligand that targets hypoxia of the overexpressed CCL-28 chemokine. b Anti-LDLR: a ligand that targets hypoxia of the marked low-density lipoprotein receptor. | ||
| Extracellular targets | Hypoxia | Modified anaerobions22–24 |
| Anti-CCL-28a,25 | ||
| Anti-LDLRb,26 | ||
| Tirapazamine27 | ||
| Acidity | Hydrolysis28 | |
| Protonation29 | ||
| Hydrophilic transformation30 | ||
| pHLIP31,32 | ||
| VEGF | Anti-VEGFmAb33 | |
| Bevacizumab34 | ||
| VEGFR | VEGFR-2 antibody35 | |
| MMP | GPLGVRGC36 | |
| PG-6 (ref. 37) | ||
| Magnetic field | Fe3O4 (ref. 38) | |
| Intracellular targets | Integrin αvβ3 | RGD39–42 |
| Integrin αvβ3 mAb43,44 | ||
| EGFR | CET45,46 | |
| Gefitinib47,48 | ||
| Erlotinib49,50 | ||
| FR | FA51–53 | |
| MTX54 | ||
| BR | Biotin55,56 | |
| TfR | Tf57,58 | |
| CD44 | HA59,60 | |
| Subcellular targets | Nucleus | NLS61 |
| TAT62 | ||
| AS1411 DNA aptamer63 | ||
| Mitochondria | TPP64,65 | |
| Cyanine cation66 | ||
| MLS67 | ||
| Lysosomes | Morpholine68 | |
| ER | Sulfamides69 | |
| Pardaxin (FAL) peptides70 | ||
1.1. “Extracellular targets”
“Extracellular targets” refers to recognition sites present in the tumor microenvironment (TME). Because of the vigorous metabolism and rapid growth of the solid tumor, extreme environments, including hypoxic environments, acidic environments, angiogenesis and overexpression of enzymes, are generated in the surrounding tumor tissues. By taking advantages of these differences between normal tissues and cancers, extensive investigations have been made for the specific treatment of tumors.71,721.2. “Intracellular targets”
“Intracellular targets” means that receptors exist on the surface of the tumor cytomembrane. Because cells are relatively separate spaces, substance ingestion requires the assistance of receptors that are embedded in the cell membrane, while specific receptors are produced to transfer different substances, including ions, small molecules and biomacromolecules. Tumor cells have abnormal metabolism, so many receptors are over-expressed. Many efforts have been focused on the development of specific drug-delivery systems based on these over-expressed receptors over the past decades.1.3. “Subcellular targets”
“Subcellular targets” means that PTAs are conjugated with organelle-targeted ligands, so the photothermal materials can efficiently induce apoptosis of organelles after endocytosis. Because of heat-sensitive proteins and genetic material in organelles, organelles are easily destroyed and cause cell death at low-dose irradiation, thereby reducing damage to surrounding normal tissues. Hence, organelle-targeted imaging and therapy have attracted wide attention in exploring technologies for tumor inhibition and life regulation.64,75,762. Dual-targeted photothermal agents
DTPTAs have got great advances over the past few years, and many materials including noble metal materials, transition metal oxide/sulfide materials, carbon materials, cyanine dyes, and organic polymers as well as other materials that have been used for designing novel PTAs for tumor ablation (Table 2).| PTAs | Irradiationa | Photothermal effecta | Targeted moieties | Specific sites or stimulation | Animal models | Ref. |
|---|---|---|---|---|---|---|
| a Photothermal performance in vitro. b AuNS: gold nanostars. c Au NRs: gold nanorods. d cRGD: cyclic RGD. e INOP-20: 20 nm INOPs. f MG: magnetic graphene. g IP: interleukin-13-based peptide. h FNPs: fluorescence NPs. i Bio: biotin. j Pi: phosphate ester group. k PM: platelet membrane. | ||||||
| Au nanoprisms | 808 nm, 500 mW cm−2, 20 min | ΔT = ∼16 °C (30 μg mL−1) | TPE@Zn and AS1411 DNA aptamers | Cell membrane and nucleus | SGC-7901 human gastric carcinoma tumor model (significant inhibition) | 63 |
| AuNSb | 808 nm, 1 W cm−2, 5 min | ΔT > 20 °C (4 μg mL−1) | HA and TPP | CD44 and mitochondrion | SCC-7 mouse squamous cell carcinoma tumor model (significant inhibition) | 78 |
| MCF-7/ADR drug resistant tumor model (significant inhibition) | ||||||
| GNS | 808 nm, 1.74 W cm−2, 4 min | ΔT = ∼15 °C (3 μg mL−1 of Au) | HA and NLS | CD44 and nucleus | 4T1 mouse breast cancer tumor model (significant inhibition) | 79 |
| 4T1 metastatic tumor model (reduced tumor metastasis) | ||||||
| AuNRsc | 808 nm, 4 W cm−2, 10 min | Increased to ∼55 °C (2 nM) | NLS and RGD | Nucleus and integrin αvβ3 | — | 80 |
| MLS and RGD | Mitochondrion and integrin αvβ3 | |||||
| RGD | Integrin αvβ3 | |||||
| GNR | 808 nm, 2 W cm−2, 4 min | Increased to 43.5 °C (40 μg mL−1) | HA and RGD | CD44 and integrin αvβ3 | — | 81 |
| GNR | 808 nm, 2 W cm−2, 10 min | ΔT = ∼30 °C (20 μg mL−1 of Au) | HA and FA | CD44 and FR | MCF-7 human breast cancer tumor model (complete elimination) | 82 |
| GNR | 808 nm, 4 W cm−2, 10 min | Increased to ∼51.6 °C | cRGDd and FA | Integrin αvβ3 and FR | B16-F10 mouse melanoma tumor model (significant inhibition) | 83 |
| GNR | 808 nm, 2 W cm−2, 10 min | Increased to ∼55 °C (20 μg mL−1 of Au) | Anti-HER2 antibody and HA | HER2 and CD44 | MCF-7 human breast cancer tumor model (complete elimination without reoccurrence) | 84 |
| Au shell | 808 nm, 1 W cm−2, 5 min | ΔT = ∼30 °C (1 mg mL−1) | Fe3O4 and FA | Magnetic field and FR | — | 85 |
| Au shell | 808 nm, 1 W cm−2, 10 min | ΔT = ∼30 °C (40 μM) | Fe3O4 and MTX | Magnetic field and FR | 4T1 mouse breast cancer tumor model (complete elimination) | 86 |
| AuNRsc | 808 nm, 2 W cm−2, 4 min | ΔT > 20 °C (1 mg mL−1) | HA and SM | CD44 and acidity | MDA-MB-231 human breast cancer tumor model (almost complete suppression) | 87 |
| Au@Pt NPs | 808 nm, 1.2 W cm−2, 10 min | ΔT = ∼65 °C (50 μg mL−1) | FA and TPP | FR and mitochondrion | — | 88 |
| CuS NPs | 980 nm, 1.5 W cm−2, 10 min | Increased to 53 °C | RGD and TAT | Integrin αvβ3 and nucleus | HeLa human cervical cancer tumor model (intratumoral/intravenous injection, complete obliteration) | 89 |
| HeLa recurrent tumor model (no recurrence) | ||||||
| WSSe | 808 nm, 0.8 W cm−2, 10 min | ΔT = ∼42 °C (240 μg mL−1) | TPP and MCF-7 cell membrane | Mitochondrion and MCF-7 cell | MCF-7 human breast cancer tumor model (complete obliteration without recurrence) | 90 |
| Fe3O4 | 808 nm, 3 W cm−2, 500 s | Increased to ∼63 °C (100 μg mL−1 of IONP-20e) | Tf and TAT | TfR and nucleus | A549 human lung cancer tumor model (significant inhibition with a slow tumor growth) | 91 |
| SCDs | 808 nm, 4 W cm−2, 10 min | ΔT = ∼33 °C (10 mg mL−1) | RGD and MLS | Integrin αvβ3 and mitochondrion | — | 67 |
| MGf | 808 nm, 6 W cm−2, 5 min | Increased to 50 °C (30 mg L−1) | Fe3O4 and IPg | Magnetic field and glioma | — | 92 |
| MGO | 808 nm, 2.5 W cm−2, 3 min | Increased to ∼50 °C (1 mg mL−1) | Fe3O4 and CET | Magnetic field and EGFR | CT-26 murine colonic carcinoma cancer tumor model (significant inhibition) | 93 |
| ICG | 808 nm, 1.54 W cm−2, 5 min | Increased to ∼61.5 °C (50 μg mL−1) | cRGDd and FA | Integrin αvβ3 and FR | — | 94 |
| ICG | 808 nm, 2 W cm−2, 5 min | Increased to 55.2 °C (6.53 μg mL−1 of ICG) | Fe3O4 and HA | Magnetic field and CD44 | U87MG human primary glioblastoma cancer tumor model (significant inhibition) | 95 |
| ICG | 808 nm, 3 W cm−2, 100 s | Increased to 78.2 °C (40 μM) | RC-12 and PG-6 | Integrin αvβ3 and MMP-2 and MMP-9 | — | 37 |
| ICG | 808 nm, 1 W cm−2, 5 min | ΔT = ∼58 °C (60 μg mL−1) | FA and TPP | FR and mitochondrion | — | 96 |
| IR825 and carbon-derivatized FNPsh | 808 nm, 2 W cm−2, 5 min | Increased to 65 °C (1 mg mL−1) | FA and TPP | FR and mitochondrion | — | 97 |
| IR825 | 808 nm, 0.8 W cm−2, 10 min | ΔT = ∼52 °C (500 μg mL−1) | Bevacizumab and IR825 | VEGF and mitochondrion | C643 human anaplastic thyroid carcinoma tumor model (complete obliteration without recurrence) | 34 |
| Bio -PPh 3 -PT | 635 nm, 0.5 W cm−2, 10 min | ΔT = ∼25 °C (0.5 mM) | Biotin and TPP | BR and mitochondrion | 4T1 mouse breast cancer tumor model (excellent inhibition) | 55 |
| ETP | 650 nm, 0.5 W cm−2, 5 min | ΔT = ∼32.8 °C (100 μM + ALP 1 h) | Pij and TPP | ALP and mitochondrion | PC-3 human prostate cancer tumor mode (almost complete suppression) | 98 |
| PDA | 808 nm, 0.8 W cm−2, 6 min | ΔT = ∼19 °C (0.2 mg mL−1) | Fe3O4 and TPP | Magnetic field and mitochondrion | B16-F10 mouse melanoma tumor model (significant inhibition with a slow tumor growth) | 99 |
| PDA and ICG | 785 nm, 0.5 W cm−2, 500 s | ΔT = ∼10 °C (50 μg mL−1) | Tf and TPP | TfR and mitochondrion | A549 human lung cancer tumor model (complete obliteration) | 100 |
| FNPs-PDA | 808 nm, 2 W cm−2, 5 min | ΔT = ∼25 °C | HA and TPP | CD44 and mitochondrion | — | 101 |
| MPDA | 808 nm, 1 W cm−2, 5 min | ΔT = ∼38 °C (100 μg mL−1) | HA and MTX | CD44 and FR | 4T1 mouse breast cancer tumor model (complete obliteration) | 102 |
| SP | 808 nm, 0.8 W, 10 min | ΔT = ∼65 °C (10 μg mL−1) | FA and cRGD | FR and integrin αvβ3 | U87MG human primary glioblastoma cancer tumor model (promising suppression) | 103 |
| PB | 808 nm, 2 W cm−2, 5 min | ΔT = ∼30 °C (1 mg mL−1) | Fe3O4 and HA | Magnetic field and CD44 | S180 mouse sarcome tumor model (almost complete suppression) | 104 |
| V2C | 1064 nm, 0.96 W cm−2, 10 min | Increased to 59.6 °C (400 μg mL−1) | RGD and TAT | Integrin αvβ3 and nucleus | MCF-7 human breast cancer tumor model (significant inhibition without recurrence) | 105 |
| Aza-BODIPY | 730 nm, 1 W cm−2, 12 min | ΔT = ∼28 °C (50 μg mL−1) | FA and TPP | FR and mitochondrion | HeLa human cervical cancer tumor model (complete suppression) | 106 |
| Melanin | 808 nm, 1.5 W, 10 min | ΔT = ∼14 °C (100 μg mL−1) | RGD and PMk | Integrin αvβ3 and tumor vasculature | MDA-MB-231/ADR human breast cancer drug resistant tumor model (significant inhibition without metastasis) | 107 |
2.1. DTPTAs based on noble metal materials
Noble metal nanoparticles (NPs) are well-studied inorganic PTAs because of their excellent photothermal and optical properties. Due to localized surface plasmon resonance (LSPR), they exhibit great talents for absorbing laser to reach electron excited state and release heat via nonradiative decay.Gold-based NPs, as excellent PTAs with intense LSPR, have got diverse applications via the modification of resonance wavelength. Researchers have developed a variety of gold nanostructures, such as gold nanorod (GNR), gold nanoshells, gold nanoprisms and gold nanostars (GNS).
Although Au NPs can passively accumulate in tumors via enhanced permeability and retention effect, the efficiency of material utilization is low. Due to the convenience of surface modification, Au NPs can be functionalized with active targeting ligands (e.g., antibodies, aptamers and peptides) to realize selective accumulation in tumor tissues.
Recently, Sun's group successfully prepared and characterized dual-targeted gold nanoplatforms (Au-Apt-TPE@Zn, Apt: aptamer), which were used to identify early apoptotic cells and perform accurate PTT for tumors (Fig. 1a).63 In their study, gold nanoprisms were coupled with phenanthroline derivatives (functionalized with tetraphenylethene (TPE)), and further stabilized with target peptide aptamers through Au–S bonds to obtain Au-Apt-TPE. The remaining nitrogen atoms of Au-Apt-TPE effectively chelated with Zn2+ to synthesize Au-Apt-TPE@Zn. Moreover, Au-Apt-TPE@Zn selectively recognized early apoptotic cells and bound cell membrane. Connected to AS1411 DNA aptamers, Au-Apt-TPE@Zn specifically targeted the nucleus. The dual-targeted therapeutic agent for cell membrane and nuclei showed prominent photothermal conversion efficiency (PCE) and allowed the nanoplatform to achieve accurate treatment for tumor inhibition.
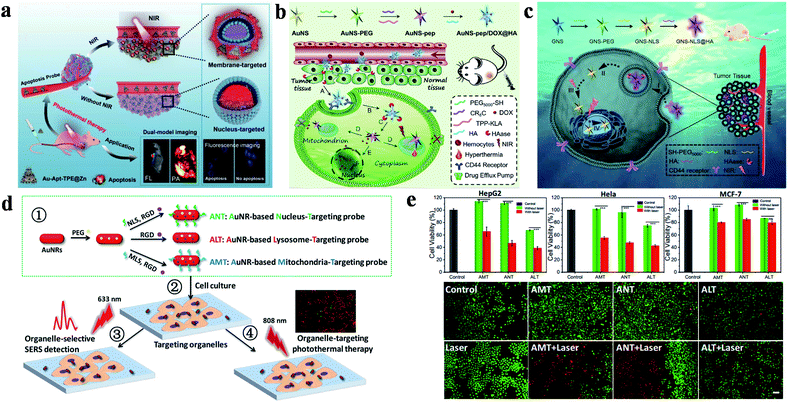 | ||
| Fig. 1 (a) Schematic diagram of Au-Apt-TPE@Zn for dual-model imaging and dual-targeted cancer PTT. Reproduced from ref. 63 with permission from Zhang et al. (b) Schematic diagram of the preparation and targeting principles of AuNS-pep/DOX@HA. Reproduced from ref. 78 with permission from Elsevier Ltd, copyright 2016. (c) Schematic diagram of the preparation and targeting principles of the cellular/intranuclear dual-targeting nanoplatform GNS-NLS@HA. Reproduced from ref. 79 with permission from the Royal Society of Chemistry, copyright 2018. (d) Schematic diagram of the preparation and application of AuNR-based nanoprobes. (e) The effect of AuNR-based nanoprobes on cell viability. Adapted from ref. 80 with permission from the American Chemical Society, copyright 2018. | ||
In addition, a dual-targeted photothermal material for targeting tumor cells and mitochondria was also studied by Zhang's group (Fig. 1b).78 AuNS-pep/DOX@HA (pep: peptide) was composed of cationic peptide R8, TPP modified α-helical pro-apoptotic peptide (TPP-KLA), doxorubicin (DOX) and HA. The nanoplatform was internalized into tumor cells through CD44 receptor-mediated recognition. TPP-KLA was used to specifically insert and destroy mitochondrial membranes, and then induce dysfunction of the mitochondria and cause mitochondrial-dependent apoptosis. In vitro and in vivo experiments showed that AuNS-pep/DOX@HA presented prominent non-resistant or resistant tumor inhibition. In 2018, the authors also designed a cellular/intranuclear dual-targeted nanoplatform for tumor therapy via PTT (Fig. 1c).79 NLS peptides were used to modify GNS to give GNS-NLS for targeting the nucleus. Subsequently, HA was coated on the surface of GNS-NLS (GNS-NLS@HA) through electrostatic interactions, which then bound with CD44 to enhance internalization and then expose NLS after degradation by hyaluronidase (HAase). GNS-NLS@HA showed high photothermal conversion (ΔT = ∼15 °C, 808 nm, 1.74 W cm−2, 4 min) and prominent tumor suppression efficiency.
Moreover, Xu and co-workers explored the therapeutic effects of GNRs in different subcellular organelles with the modification of specific targeting peptides and RGD peptides (Fig. 1d).80 Surface-enhanced Raman scattering spectroscopy promoted AuNRs to achieve super-resolution imaging of biomolecules and monitor the position of therapeutic agents. Under the same experimental conditions, AMT (AuNRs modified with MLS and RGD) or NLS (AuNRs modified with NLS and RGD) have better tumor killing effect than ALT (AuNRs only modified with RGD) (Fig. 1e).
Another effective dual-targeted model is the combination of two different tumor cell-targeted ligands such as RGD, HA and FA. Recently, Xu and co-workers reported a mesoporous silica (mSiO2)-coated nanoplatform (DOX-GNRs@mSiO2-HA-RGD) by combining HA, RGD and DOX (Fig. 2a).81 Cell uptake studies proved that the internalization of the dual-targeted DOX-GNRs@mSiO2-HA-RGD was more effective than single-targeted and untargeted therapeutic agents. In addition, DOX-GNRs@mSiO2-HA-RGD showed excellent photothermal conversion properties, with a rapid increase to 43.5 °C (808 nm, 2.00 W cm−2, 4 min). Yao and co-workers designed a pH and near-infrared (NIR) dual-responsive drug release nanoplatform for the cooperative chemo-photothermal therapy of breast cancer, which was prepared by combining GNR, FA, HA, dopamine, adipic acid dihydrazide and DOX (Fig. 2b).82 Compared with the non-FA-modified NPs, the dual-responsive nanoplatform was more efficient to deliver GNR and DOX to MCF-7 cells. Moreover, tumor cells were killed more effectively through inducing apoptosis under NIR irradiation. Recently, Li and co-workers also studied the therapeutic effect of DOX-loaded FA/RGD dual-targeted GNR (denoted as FA/RGD-DOX-hz-GNRs, hz: a pH-sensitive hydrazone) for B16-F10 xenograft tumors.83In vivo experiments showed that this dual-targeted nanoplatform exhibited excellent tumor therapeutic effects via the synergistic cooperation of DOX-induced apoptosis, heat induced necrosis and angiogenesis inhibition. Other than that, human epidermal growth factor receptor 2 (HER2) is also an important targeting site for tumor therapy, which is over-expressed in multiple tumors. Conjugated with HER2, a pH, GSH and HAase triple-responsive nanoplatform GNR-HA-ALA/Cy7.5-HER2 (ALA: 5-aminolevulinic acid) was reported by Yao and co-workers.84In vivo experiments illustrated that tumor tissues could be completely eliminated without obvious side effects. This nanoplatform provided a potential strategy for HER2/CD44 targeted breast cancer PDT/PTT.
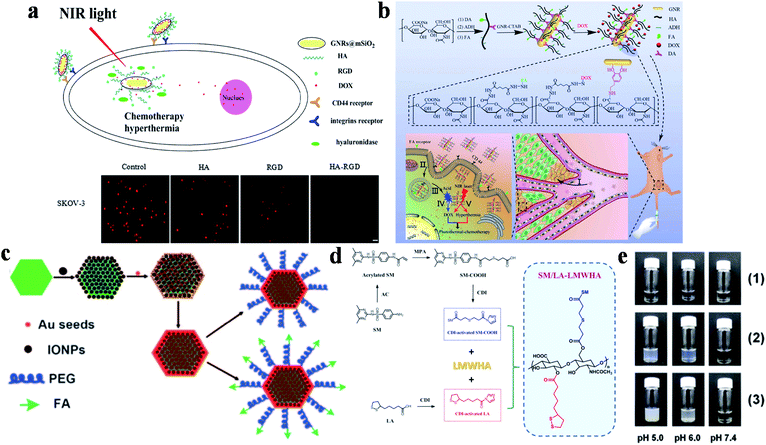 | ||
| Fig. 2 (a) Schematic diagram of DOX-GNRs@mSiO2-HA-RGD for targeted therapy and cell uptake studies of DOX-GNRs@mSiO2-HA-RGD with different inhibitors (HA, RGD and HA-RGD). Adapted from ref. 81 with permission from Elsevier B.V., copyright 2017. (b) Schematic diagram of the preparation and targeting principles of GNRs-HA-FA-DOX. Adapted from ref. 82 with permission from the American Chemical Society, copyright 2017. (c) Schematic diagram of the preparation method of MFNPs. Reproduced from ref. 85 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2011. (d) Schematic diagram of the synthesis of SM/LA-LMWHA. (e) The effects of different pH on (1) LMWHA, (2) SD/LA-LMWHA, and (3) SM/LA-LMWHA. Reproduced from ref. 87 with permission from Elsevier Ltd, copyright 2019. | ||
Furthermore, dual-targeted therapy can also be achieved via simultaneously targeting the tumor environment and tumor cells. Liu's group modified ultrasmall superparamagnetic IONPs onto NaYF4-based upconversion NPs, and then coated a thin gold shell through seed-induced reduction growth, on which poly(ethylene glycol) (PEG) were anchored as linkers to conjugate FA to prepare the multifunctional platform (denoted as FA-PEG-MFNP) (Fig. 2c).85 FA-PEG-MFNP could achieve dual-modal targeting capability mediated by magnetic field and FR with the monitoring of upconversion luminescence and MR imaging. After that, Zhang's group constructed a MTX-Fe3O4-gold shell (MFG) nano-system hybridized with LPM lipoic acid (LA)-PEG-MTX (LPM) to obtain MFG-LPM NPs for dual-targeted chemo-photothermal therapy.86 MTX has a similar structure to FA, which can be used for cancer therapy as both targeted ligand and chemotherapeutic drug. This dual-targeted MFG-LPM had excellent healing talent that cured all tumor-bearing mice in a short time, while mice in none-magnetic group needed a second injection therapy.
The acidic microenvironment of tumor tissues has also been used for dual-targeted cancer therapy. Lee's group combined low molecular weight hyaluronic acid (LMWHA), the pH-sensitive group sulfamethazine (SM) and GNR to synthesize dual-targeted therapeutic platforms from ligand-mediated cell uptake and acid-induced aggregation (Fig. 2d).87 When the pH was lower than 7.4, the de-ionization of SM in AuNRs@SM/LA-LMWHA improved the surface hydrophobicity, resulting in particle aggregation and gradually increasing size (Fig. 2e). And then, the NPs were internalized into tumor cells through CD44 receptor-mediated recognition for tumor therapy.
Other noble metals (e.g., platinum) are also used as DTPTAs for enhanced tumor therapy by taking the advantages of photothermal stability and certain catalytic ability. In 2017, Lin's group developed Au@Pt NPs modified with a cell-targeting ligand FA and a mitochondria-targeting group TPP for PDT/PTT synergistic therapy.88 In this nanoplatform, the excellent catalytic activity of Pt NPs could enhance the peroxidase-like catalysis of H2O2 to improve the efficacy of PDT.
2.2. DTPTAs based on transition metal oxide/sulfide materials
Although noble metal NPs such as gold and platinum have shown great potential applications in the field of tumor photothermal treatment, the high cost, difficult biodegradation and toxicity during long-term metabolic processes in the body have restricted further research and clinical practice application. Transition metal oxide/sulfide NPs as another type of inorganic PTAs exhibit strong NIR-absorption, low cost and high PCE, which has attracted much attention in PTT for tumor tissues. Because of these advantages, copper sulfide (CuS) NPs are widely used in the treatment of multifarious diseases such as cancer,108,109 atherosclerosis110 and diabetes,111 and show excellent biological applications based on low cytotoxicity and controllable morphology. Tang and co-workers developed a nucleus-targeted PTT strategy based on CuS NPs to achieve intra-nuclear PTT and effectively prevent local cancer recurrence (Fig. 3a).89 Through the surface modification of mesoporous silica coated CuS (CuS@MSN) by RGD and TAT peptides, CuS@MSN-TAT-RGD NPs directly targeted tumor cells and further entered the nucleus. After irradiation with a 980 nm NIR laser, the endonuclear genetic materials were destroyed by hyperthermia, resulting in cell necrosis. Under 980 nm laser irradiation for 5 minutes, the cell killing rate of the NPs with targeting ability was 84%, while that of non-targeting NPs was 42% (Fig. 3b). This PTT platform, with the capability of targeting nucleus, provides powerful possibilities for tumor ablation and anti-recurrence.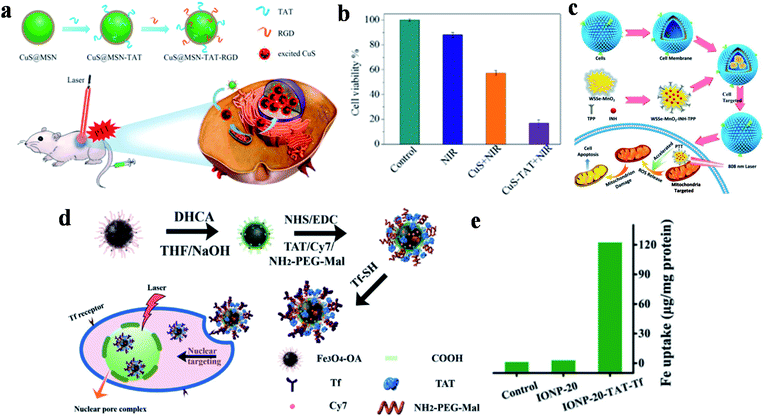 | ||
| Fig. 3 (a) Schematic diagram of the preparation and targeting principles of CuS@MSN-TAT-RGD NPs. (b) The effect of different treatment conditions on cell viability. Reproduced from ref. 89 with permission from the American Chemical Society, copyright 2018. (c) Schematic diagram of the preparation and treatment principles of WSSe/MnO2-INH-TPP@CM. Reproduced from ref. 90 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2019. (d) Schematic diagram of the preparation and targeting principles of IONP-TAT-Tf. (e) Nuclear iron elemental quantification of IONP-20 and IONP-20-TAT-Tf by A549 cells. Reproduced from ref. 91 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2017. | ||
WSSe nanosheets were also used in dual-targeted PTT for tumors by Zhang's group via the preparation of a WSSe/MnO2-INH nanocomposite (Fig. 3c).90 In this study, Mn2+ generated by the degradation of MnO2 was used to catalyze the isoniazid (INH) to obtain hydroxyl radicals (˙OH) for achieving PTT and PDT anticancer treatment. WSSe/MnO2-INH-TPP@CM (CM: cell membrane) was coated with cancer cell membrane extracted from the breast cell line MCF-7, and then it was modified with the mitochondrial-targeted group TPP to realize dual-targeted tumor therapy. WSSe/MnO2-INH-TPP@CM showed good anti-cancer effects both in vivo and in vitro.
Moreover, Yang and co-workers developed nucleus-targeted multifunctional magnetic NPs (IONP-20-TAT-Tf) bearing Tf and TAT peptides (Fig. 3d).91 The conjugated monodisperse magnetic NPs exerted considerable photothermal stability and high PCE (∼37%). Quantitative analysis showed that the accumulation of multifunctional IONP in the nucleus was 45-fold higher than that without TAT modification (Fig. 3e). During in vivo experiments, the composite NPs effectively inhibited xenografted tumors irradiated by NIR lasers, and are expected to be used in cancer treatment.
2.3. DTPTAs based on carbon materials
Carbon-based nanomaterials have great potential applications in the field of tumor PTT. Graphene oxide, carbon dots and other carbon-based materials have extensive optical absorption and reasonable photothermal properties, which has aroused widespread interest. Carbon nanodots have outstanding characteristics, such as high photostability, low toxicity, high solubility, low cost and good biocompatibility. However, the low efficiency of the absorption in the visible to NIR window limits their application in light-sensitive cancer treatment strategies and in vivo imaging. Xu's group prepared a new type of super carbon dots (SCDs) (about 20 nm) with broad absorption from the visible light to NIR region.67 The carbon dots (CDs) possess a high PCE for PTT, and can be functionalized with dual-targeted ligands (RGD and MLS) for cancer cells and mitochondria. The SCDs were constructed via the self-assembly of small sized CDs (around 5 nm) in an acidic environment, which could be located precisely in the mitochondria of cancer cells for cancer therapy (Fig. 4a and b). The experimental results showed that the difference in the survival rate between the tumor and normal cells was as high as 70%, reflecting the high specificity and selectivity of these SCDs (Fig. 4c and d).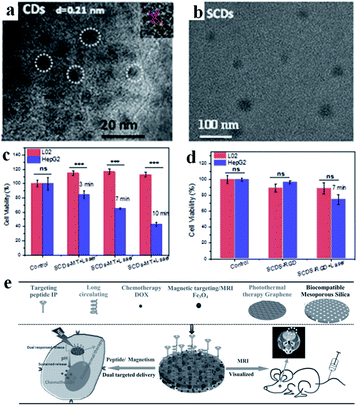 | ||
| Fig. 4 TEM images of (a) CDs and (b) SCDs. Cell viabilities of L02 cells and HepG2 cells treated with (c) SCDs-MT and (d) SCDs-RGD and irradiated for 7 min. Adapted from ref. 67 with permission from Elsevier Ltd, copyright 2019. (e) Schematic diagram of the preparation and application of MGMSPID. Reproduced from ref. 92 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2013. | ||
Due to its large specific surface area, excellent electrical properties and optical properties, graphene has attracted extensive attention in many fields in recent years. Graphene oxide can be obtained via the chemical oxidation of graphene, which has a good PCE for PTT. Kong's group successfully prepared multifunctional magnetic graphene as a nanomedical platform (denoted as MGMSPID) for MRI guided chemo-photothermal glioma treatment (Fig. 4e).92 This photothermal agent could be easily prepared via a PEGylated method and then modified with an interleukin-13-based peptide (IP). In addition, the MGMSPID system showed thermal stimulation, pH-response and sustained release properties. All these features provide a powerful multi-functional therapeutic platform for visual glioma treatment. Chen's group also developed a pH-sensitive dual-targeted magnetic nanocarrier for chemo-phototherapy in cancer treatment.93 The authors prepared magnetic graphene oxide (MGO) via chemical coprecipitation, and then modified MGO with PEG and CET to obtain MGO-PEG-CET. MGO-PEG-CET was used for magnetic and receptor-mediated dual-targeted synergistic treatment to delivery DOX to EGFR over-expressed tumor cells. In vitro cytotoxicity tests showed that the half-maximum inhibitory concentration (IC50) value of the dual-targeted nanoplatform MGO-PEG-CET/DOX on CT-26 cells was 1.48 μg mL−1, which was lower than the group of MGO-PEG/DOX (2.64 μg mL−1).
2.4. DTPTAs based on cyanine dyes
Compared with inorganic PTAs, organic PTAs have the inherent advantages of biocompatibility and degradability, among which cyanine dyes are the most representative agents, which have widespread applications. Indocyanine green (ICG), one of the cyanine dyes, has drawn a lot of attention due to its great biosafety, and has been approved by the Food and Drug Administration for clinical fluorescence imaging.112 ICG also shows great properties for tumor PTT, PDT and fluorescence imaging, with satisfying water solubility and potential mitochondrial targeting, as well as splendid PCE properties. However, since ICG is easily cleared in vivo and has a short cycle time, it is usually administered via a delivery system. Obviously, a dual-targeted drug delivery system is an ideal strategy to carry ICG to tumor tissues for imaging and therapy. In 2018, Wang et al. developed thermo-sensitive NPs to drive ICG, photosensitizer chlorin e6 (Ce6) and chemotherapy drug cisplatin into cancer cells with the help of tumor recognition groups.94 Targeted FA molecules and cRGD peptides were covalently anchored to different amphiphilic polymers and the modified polymers self-assembled to form NPs to enhance the active targeting properties.This intelligent drug release realized synergistic treatment and ablated tumors under NIR irradiation. To improve the cancer-targeted efficiency, Anilkumar et al. constructed dual targeted magnetic photosensitive liposomes (MPLs) to deliver ICG to tumor tissues driven by a magnetic field and to internalize drugs into cells via the combination of HA and CD44.95 In this study, citric acid-coated Fe3O4 magnetic NPs (CMNs) and ICG molecules were loaded in MPLs via a solvent evaporation/hydration technique, and then HA-PEG was connected by electrostatic interaction triggered self-assembly to fabricate HA-PEG-MPLs. These HA-PEG-MPLs particles not only achieved tumor aggregation enhanced therapy, but also increased the PCE (ΔT = ∼30 °C, 808 nm, 2.0 W cm−2, 5 min) via the combined influence of ICG and CMNs. Therefore, HA-PEG-MPLs showed superior inhibiting ability in a xenograft tumor model. Fang et al. constructed biocompatible selenium NPs (SeNPs) to deliver DOX and ICG to tumor cells (Fig. 5a).37 In order to assure agents arrived in tumor cells successfully, chitosan was chosen as a linker to conjugate with RGD-derived RC-12 peptide and MMP-recognized PG-6 peptide to give SeNPs-DOX-ICG-RP. The internalization of tumor cells triggered by endocytosis offers great possibilities of accurate treatment.
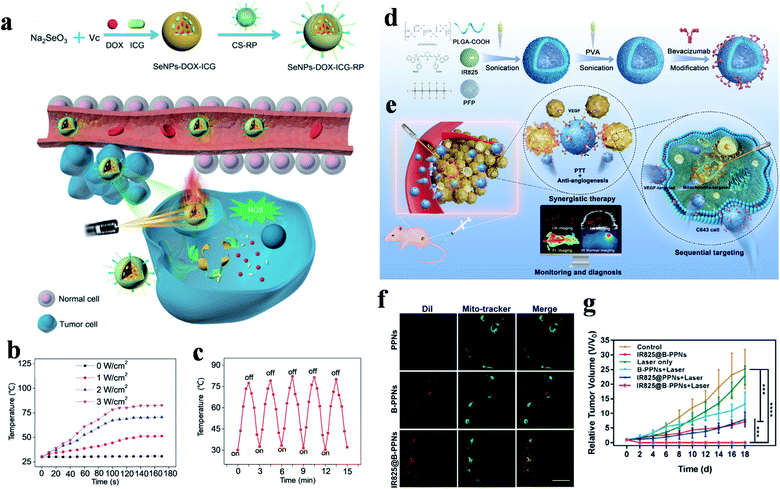 | ||
| Fig. 5 (a) Preparation and delivery mechanisms of SeNPs-DOX-ICG-RP with NIR laser irradiation. (b) Temperature changes of SeNPs-DOX-ICG-RP under different irradiation powers. (c) Photothermal stability experiment. Adapted from ref. 37 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2018. (d) Synthesis and (e) sequential targeting process of IR825@B-PPNs with monitoring and diagnosis. (f) CLSM images of mitochondrial localization in C643 cells. The scale bar is 20 μm. (g) Tumor growth curves after treatment with different therapies. Adapted from ref. 34 with permission from Acta Materialia Inc, published by Elsevier Ltd, copyright 2019. | ||
Besides this, the Se nanosystems showed amazing PCEs and temperatures that could increase to 78.2 °C (808 nm, 3.0 W cm−2, 100 s), and the excellent stability was also certified via a laser turn on/off experiment five times (Fig. 5b and c).
Wu et al. constructed a tumor and mitochondria dual-targeted nanocarrier for mitochondrial locating PTT and chemotherapy.96 In this case, FA and TPP were respectively connected to amphiphilic polymer DSPE-PEG, and the resulting polymers were mixed and self-assembled to form nanovesicles for encapsulating ICG and DOX. The vesicles displayed great colloid stability and released DOX via the rapid temperature rise (ΔT = ∼58 °C, 808 nm, 1.0 W cm−2, 5 min) induced by NIR irradiation. Importantly, combined with FA and TPP, these nanoagents showed powerful mitochondria locating capability (p up to 0.84) and enhanced cytotoxicity, with a sharply reduced MCF-7 cell survival rate of as low as 3.8%.
IR 825 is a kind of heptamethine type NIR dye with perfect photothermal conversion performance. Despite possessing a similar structure to ICG, IR 825 has limited applications in the theranostic field due to low water solubility, so it is usually delivered to tumor tissue via a micelle encapsulation technique. In 2017, Kang et al. described a FA and TPP modified polymeric core–shell therapeutic agent for specifically transmitting IR 825 and 3-bromopyruvate (BP) to tumor mitochondria.97 PEG grafted poly(dimethyl aminoethyl methacrylate) (PEG-g-PDMA) was connected with catechol and carbonized with sulfuric acid to obtain the core, and the shell was composed of PEG-g-PDMA, tumor-targeted FA, mitochondria-targeted TPP, IR 825 and phenylboric acid, which reacted with catechol to achieve core–shell binding. This therapeutic agent damaged tumor mitochondria and induced cell death triggered by hyperpyrexia (up to 65 °C) and the glycolysis inhibition of BP under NIR irradiation (808 nm, 2.0 W cm−2, 5 min).
Since the IR 825 molecules have lipophilicity cationic structure for mitochondrial aggregation, they are also used for mitochondrial-targeted therapy. Based on this feature, Wang et al. engineered a targeted theranostic agent (IR825@B-PPNs) for anaplastic thyroid carcinoma oncotherapy (Fig. 5d and e).34 IR 825 and perfluoropentane (PFP, an ultrasound contrast agent) were loaded in poly(lactic-co-glycolic acid) (PLGA) to form IR825@PPNs, and then, polyvinyl alcohol (PVA) and bevacizumab antibodies were modified (IR825@B-PPNs) in order to promote tumor aggregation. The conjugated bevacizumab specifically recognized VEGF and blocked the combination of VEGF and receptors. With the monitoring and diagnosis of the photoacoustic, fluorescence, and ultrasonic multi-mode imaging, tumor and mitochondrial aggregation enhanced the thermal damage of IR 825 to tumor cells. The well-merged fluorescence signals of IR825@B-PPNs and Mito-Tracker indicated that the theranostic agent had a tendency for mitochondrial localization and in vivo experiments realized the complete curing of a C643 tumor (Fig. 5f and g).
Other cyanine dyes also make a great contribution to dual-targeted PTT. In 2019, Tang and co-workers designed a novel small molecule (Bio-PPh3-PT) for highly effective and selective tumor mitochondrial PTT (Fig. 6a left).55 In this study, quinoline and indole blocks were connected via continuous double bonds to obtain a solid compound that possesses the ability of photothermal conversion, and then conjugated it with biotin and TPP for targeting tumors and mitochondria. The DTPTAs showed a superb PCE (37.8%) with a temperature change of ∼25 °C (635 nm, 0.5 W cm−2, 5 min). Biotin was used to distinguish diseased tissue from normal tissue and TPP functioned as an important targeting group for locating in mitochondria. The dual-targeted photothermal material can cause irreversible damage at a light power as low as 0.5 W cm−2. Besides this, during the treatment of tumor-bearing mice, apparent inhibition occurred in the dual-targeted group, while the therapeutic effect in the non-targeted group was negligible (Fig. 6b).
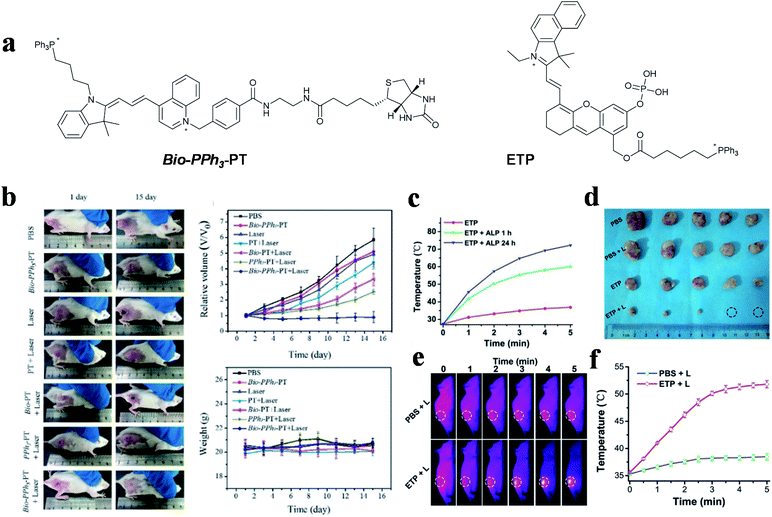 | ||
| Fig. 6 (a) Dual-targeted molecules Bio-PPh3-PT and ETP. (b) Therapeutic effect under different treatments. Adapted from ref. 55 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2019. (c) Temperature change curves of ETP treated with different concentrations of ALP under NIR laser irradiation. (d) Tumor photographs of mice. (e) Thermal imaging and (f) quantitative curves of PC-3 tumor-bearing mice. Adapted from ref. 98 with permission from the Royal Society of Chemistry, copyright 2019. | ||
The photothermal conversion capability of small molecule PTAs was regulated by the electrical changes initiated from the regulation of specific substances in tumor cells.113 An aggregation-enhanced PTT probe (termed as ETP) was developed by Yao et al. for mitochondria-targeted imaging and therapy,98 which was activated by alkaline phosphatase (ALP) (Fig. 6a right). A phosphate ester group was introduced into the body of the probe for inhibiting molecular activity. After hydrolysis by ALP, which is overexpressed in prostate cancer, molecule absorption occurred with a large redshift, to allow active FL and photoacoustic imaging. Simultaneously, the photothermal performance and therapeutic effect were enhanced via molecular aggregation and mitochondria-targeting properties. The probes showed a 32.8 °C temperature change in in vitro experiment after incubation with ALP for 24 h, while the group without incubation only changed by 9.5 °C (650 nm, 0.5 W cm−2, 5 min) (Fig. 6c). Thermal imaging showed that the tumor temperature increased to over 50 °C, which indirectly illustrated the effective tumor healing ability of the activated DTPTAs (Fig. 6d–f).
2.5. DTPTAs based on organic polymers
Organic polymers have better light stability than small molecules, which have attracted scientists' interest for PTT. Among these polymers, polydopamine (PDA) plays a crucial role in PTT due to it being in low cost and easy to synthesize. It is worth mentioning that PDA as a natural biodegradable polymer has been widely used in targeted PTT. Mitochondria-targeted PTAs were demonstrated by Wang et al. using magnetic materials and TPP as targeting ligands.99 Magnetic Fe3O4 NPs were coated with PDA to form core–shell structures, on which TPP and GSH responsive PEG were grafted. At last, DOX was further adsorbed on the surface via π–π stacking to give the desired therapeutic agent Fe@PDA-TPP/SS/DOX. This multistage targeted therapeutic agent was guided by an external magnetic field to reach tumor tissues, and experienced lysosome escape and GSH mediated degradation to expose TPP for mitochondrial aggregation. Under low power density irradiation, Fe@PDA-TPP/SS/DOX exerted an excellent temperature increase of 19 °C within 6 minutes (808 nm, 0.8 W cm−2) and released DOX to destroy mitochondrial DNA. Analogously, Guo et al. also constructed a Fe3O4@PDA@mSiO2 core–shell material for targeted PTT (Fig. 7a).100 In this study, tumor-identified Tf and mitochondria-targeted TPP were anchored on the surface of Fe3O4@PDA@mSiO2 materials to enhance their transport to tumor subcellular organelles. In addition, ICG molecules were loaded in the outermost mSiO2 layer to improve photothermal conversion and produce ROS. This composite material showed a considerable capacity of heat production (ΔT = ∼10 °C, 785 nm, 0.5 W cm−2, 500 s) under a low dose of light radiation and outstanding tumor ablation with complete healing efficacy (Fig. 7b and c).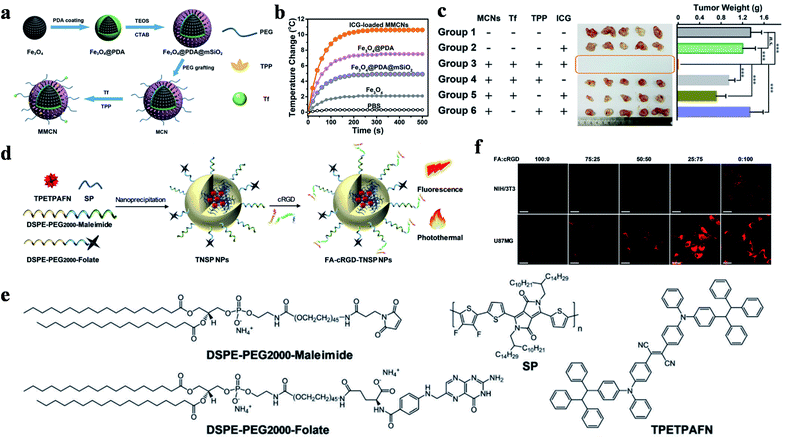 | ||
| Fig. 7 (a) Schematic illustration of the preparation of magnetic/mitochondria-targeting composite NPs. (b) The photothermal response curves of different NP solutions with an NIR laser. (c) Tumor photograph (middle) and quantitative weights (right) of the different treatment groups; reproduced from ref. 100 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2016. (d) Schematic diagram of NP preparation. (e) Chemical structures of the compositions. (f) CLSM localization images in NIH/3T3 normal cells and U87MG GBM cells incubated with different proportions of targeting ligand modified NPs. Scale bars: 50 μm. Adapted from ref. 103 with permission from the Royal Society of Chemistry, copyright 2019. | ||
After carbonizing them with concentrated sulfuric acid, PDA ramified fluorescent carbon NPs (FNPs-PDA) were synthesized by Park and co-workers.101 FNPs-PDA, acting as a PTA and imaging agent, underwent a boracic acid mediated esterification reaction to give it a HA shell, which was then modified with TPP and β-cyclodextrin (β-CD) to obtain the nanocomposite drug FNPs-PDA@HA-TPP-CD-PTX. When the therapeutic agent arrived at the tumor tissues, the combination of HA and CD44 promoted endocytosis and TPP triggered mitochondrial aggregation after lysosome escape. The low pH in the lysosome not only promoted the heat production of FNPs-PDA@HA-TPP-CD-PTX, but also facilitated the release of paclitaxel (PTX) in the β-CD hydrophobic cavity for multimodal therapy.
Zhu and co-workers reported magnetic polydopamine (MPDA), which was loaded with DOX and then coated with HA-MTX.102 In this study, the magnetic Fe3O4 NPs guided tumor aggregation in vitro in a magnetic field, while HA as an active targeting ligand enhanced CD44 mediated endocytosis. Besides this, MTX played the dual roles of chemotherapeutic drug and ligand to FR. In vitro experiments showed that tumor cells could be efficiently killed by the synergistic treatment of PTT and chemotherapy based on a high PCE (ΔT = ∼38 °C, 808 nm, 1.0 W cm−2, 5 min).
Semiconducting polymers (SP) are chain-like macromolecular materials with semiconductor properties. Due to their advantages of long wavelength absorption and efficient PCE, SP have been widely studied for PTT and photoacoustic imaging.114–120 Cai et al. used SP as a photothermal agent for glioblastoma multiforme (GBM) ablation, and investigated the influences of two targeted ligands in different ratios on tumor internalization (Fig. 7d and e).103 SP and aggregation-induced emission molecules were loaded in DSPE-PEG2000, meanwhile FA and RGD were conjugated on the surface to achieve specific tumor accumulation. In vitro and in vivo experiments showed that the SP exhibited a perfect light absorption and PCE in the NIR region, which resulted in a rapid temperature rise of ∼65 °C after 10 min of exposure (808 nm, 0.8 W). Moreover, NPs modified with FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RGD (25
RGD (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75) were proven to have superior endocytosis ability (Fig. 7f).
75) were proven to have superior endocytosis ability (Fig. 7f).
2.6. DTPTAs based on other materials
Prussian blue (PB) as an ancient pigment has also been used as a PTA to treat tumor in recent years. Taking advantage of the absorption of PB in the NIR region, a multifunctional PTA was designed and synthesized by Du et al. for dual-targeted PTT.104 In their study, PB NPs were designed as a kind of PTAs, while magnetic Fe3O4 NPs were adsorbed on the surface of upconversion NPs as magnetic targeting ligands. In addition, PEG-HA was then modified on the outermost layer to enhance the water solubility and active targeting of the material. Upon being exposed to NIR light, the composite materials rapidly increased in temperature from 20 to 50 °C in 5 min (808 nm, 2.0 W cm−2). In vivo experiments showed that they exhibited around four-fold higher aggregation in tumor than control groups without targeting ligands.The limited penetration depth of light and the thermal resistance caused by heat shock proteins significantly restricts the therapeutic efficiency of PTT. To solve these problems, Zhang's group developed a nucleus-targeting strategy for low-temperature PTT in the NIR-II region.105 The authors first modified V2C quantum dots with a nucleus-targeting TAT peptide (V2C-TAT), and then wrapped the V2C-TAT quantum dots into an endogenous exosome (Ex). Then, RGD was connected to quantum dots to obtain a cancer cell membrane and nucleus dual-targeted system (V2C-PEG-TAT@Ex-RGD). This V2C-PEG-TAT@Ex-RGD system exhibited good biocompatibility, long circulation time, endosome escape and amazing PCE in the NIR-II region. Furthermore, this type of PTA can target cells and enter the nucleus to achieve low-temperature PTT with good tumor destruction efficiency.
Boron dipyrromethene (BODIPY) has also been extensively studied due to its prominent extinction coefficient, photostability, photothermal and photodynamic capabilities. In 2013, Chen et al. described an aza-BODIPY derivative (termed as MeOABBr) for multimodal imaging and phototherapy.106 The designed MeOABBr molecule exhibited a high singlet oxygen quantum yield (up to 84%) and excellent PCE (up to 40%), which made it possible to achieve tumor suppression by phototherapy. After mixing with PEG-NH2, PEG-TPP and PEG-FA, the molecules formed NPs via self-assembly for tumor elimination. In vivo experiments showed that the synergistic effect of PTT and PDT inhibited and eliminated HeLa tumors, and presented little damage to normal tissues and organs.
Melanin, a natural polymer that exists in many organisms, has been applied as a biocompatible therapeutic agent due to its excellent NIR light absorption and PCE. Wang and co-workers encapsulated melanin NPs (MNPs) and DOX into RGD-modified platelet vesicles to create a dual-targeted drug delivery system, which was used for treating multidrug resistant cancer and inhibiting metastasis.107In vivo experiments illustrated that the growth and metastasis of resistant breast cancer were efficiently inhibited by DOX and the MNPs (ΔT = ∼14 °C, 808 nm, 1.5 W, 10 min).
3. Conclusions
In recent years, PTT based on dual-targeted ligands has attracted extensive attention, and great advances have been made in this field. In this perspective, typical targeting strategies can be divided into three parts, “extracellular targets”, “intracellular targets”, and “subcellular targets” according to the recognizing position. Compared with traditional PTAs, DTPTAs have the great advantage of being able to precisely target cancer cells or organelles. First, dual-targeted PTT shows better selectivity and decreased side effects to the surrounding tissues. Second, dual-targeted therapy makes tumor cells uptake DTPTAs more easily to improve therapy efficiency.Although great efforts have been made to develop efficient DTPTAs, there are still some challenging problems to be resolved in the future. (a) Photosensitizers: the PCE and potential biological toxicity of photosensitizers should be carefully considered in advance. For examples, inorganic PTAs are known to have higher PCEs in general, but the degradation in vivo is still unclear. Although organic PTAs have inherent excellent biodegradability, they also suffer from the limitations of photostability and thermal stability. Besides this, PTAs that strongly absorb in the NIR-II region show superior potential due to their deep tissue penetration and high maximum permissible energy for the skin. For example, semiconducting polymers improve the possibility of clinical application.121 (b) Configuration choice: it has been reported that NPs of around 100 nm in size have a longer circulating time in vivo, meaning that it is difficult for them to clear biological barriers. Moreover, rod-shaped materials have better permeability in the tumor tissue, which is beneficial for the treatment of solid tumors. Many investigations have illustrated that organic PTAs should be synthesized into nanoscale materials. (c) Targeting ligands: given the fact that targeting ligands have different compositions and aiming positions, it is necessary to reasonably design PTAs with targeting ligands. A synergistic targeting strategy is an ideal method by which to prepare specific PTAs, however, targeting ligands should not interfere with each other to identify tumor sequentially. It is worth noting that large ligands can shield small molecule ligands resulting in decreased targeting efficiency, so this should be avoided before the design of the multifunctional PTAs. (d) Methods for the modification of targeting ligands: the methods for the modification of targeting ligands also seriously influences the efficiency of dual-targeted PTAs for cancer therapy. In general, multifunctional PTAs can be simply coated onto a targeted delivery system, which can easily cause side effects because of the risk of leakage. So, it is highly desirable to connect targeting ligands to PTAs using covalent bonds. Although synthetic methods may be more difficult than physical coating, they provides a safe strategy for in vivo therapy. In order to achieve optimal tumor delivery, in the preparation of an ideal dual-targeted agent the above problems should be considered in advance.
In conclusion, recent advances in dual-targeted PTT for tumors are summarized in this review. DTPTAs have provided an effective and non-invasive alternative with more accurate positioning capabilities compared to traditional PTAs, which possess the extensive advantages of low side effects and high efficiency in tumor therapy. Although scientists have made great efforts in this field, there are still many challenges to overcome before realizing clinical applications. Prospectively, good biocompatible DTPTAs have low toxicity to organisms, and will be superior choices for clinical application. We believe that this report will provide valuable ideas and references for researchers to enable them to design novel DTPTAs to enhance cancer therapy.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the Key Research and Development Program of Shandong Province (2018YFJH0502), the National Natural Science Foundation of China (21535004, 91753111, 21927881, 21874086, 21602125 and 21775094), the National Key R&D Program of China (2019YFA0210100), and the Youth Innovation Science and Technology Program of Higher Education Institution of Shandong Province (2019KJC022).Notes and references
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2019, 69, 7–34 CrossRef PubMed.
- K. D. Miller, L. Nogueira, A. B. Mariotto, J. H. Rowland, K. R. Yabroff, C. M. Alfano, A. Jemal, J. L. Kramer and R. L. Siegel, Ca-Cancer J. Clin., 2019, 69, 363–385 CrossRef PubMed.
- P. Wang, S. Sun, H. Ma, S. Sun, D. Zhao, S. Wang and X. Liang, Mater. Sci. Eng., C, 2020, 108, 110198 CrossRef CAS PubMed.
- Y. Chen, H. Zhong, J. Wang, X. Wan, Y. Li, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 5773–5778 RSC.
- G. Song, C. Liang, H. Gong, M. Li, X. Zheng, L. Cheng, K. Yang, X. Jiang and Z. Liu, Adv. Mater., 2015, 27, 6110–6117 CrossRef CAS PubMed.
- Z. Deng, N. Wang, Y. Liu, Z. Xu, Z. Wang, T. C. Lau and G. Zhu, J. Am. Chem. Soc., 2020, 142, 7803–7812 CrossRef CAS PubMed.
- E. Perez-Herrero and A. Fernandez-Medarde, Eur. J. Pharm. Biopharm., 2015, 93, 52–79 CrossRef CAS PubMed.
- W. Fan, P. Huang and X. Chen, Chem. Soc. Rev., 2016, 45, 6488–6519 RSC.
- Y. Shen, A. J. Shuhendler, D. Ye, J. J. Xu and H. Y. Chen, Chem. Soc. Rev., 2016, 45, 6725–6741 RSC.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- L. Zhao, Y. Liu, R. Xing and X. Yan, Angew. Chem., Int. Ed., 2019, 59, 3793–3801 CrossRef PubMed.
- M. Kim, J. H. Lee and J. M. Nam, Adv. Sci., 2019, 6, 1900471 CrossRef PubMed.
- X. Duan, C. Chan, N. Guo, W. Han, R. R. Weichselbaum and W. Lin, J. Am. Chem. Soc., 2016, 138, 16686–16695 CrossRef CAS PubMed.
- B. Feng, F. Zhou, B. Hou, D. Wang, T. Wang, Y. Fu, Y. Ma, H. Yu and Y. Li, Adv. Mater., 2018, 30, 1803001 CrossRef PubMed.
- C. S. Chiang, Y. J. Lin, R. Lee, Y. H. Lai, H. W. Cheng, C. H. Hsieh, W. C. Shyu and S. Y. Chen, Nat. Nanotechnol., 2018, 13, 746–754 CrossRef CAS PubMed.
- L. Du, H. Qin, T. Ma, T. Zhang and D. Xing, ACS Nano, 2017, 11, 8930–8943 CrossRef CAS PubMed.
- J. Son, G. Yi, J. Yoo, C. Park, H. Koo and H. S. Choi, Adv. Drug Delivery Rev., 2019, 138, 133–147 CrossRef CAS PubMed.
- N. Platet, A. M. Cathiard, M. Gleizes and M. Garcia, Crit. Rev. Oncol. Hematol., 2004, 51, 55–67 CrossRef PubMed.
- J. A. Koenig and J. M. Edwardson, Trends Pharmacol. Sci., 1997, 18, 276–287 CrossRef CAS PubMed.
- Y. Wen, C. L. Schreiber and B. D. Smith, Bioconjugate Chem., 2020, 31, 474–482 CrossRef CAS PubMed.
- W. Chen, Y. Wang, M. Qin, X. Zhang, Z. Zhang, X. Sun and Z. Gu, ACS Nano, 2018, 12, 5995–6005 CrossRef CAS PubMed.
- C.-H. Luo, C.-T. Huang, C.-H. Su and C.-S. Yeh, Nano Lett., 2016, 16, 3493–3499 CrossRef CAS PubMed.
- F. Chen, Z. Zang, Z. Chen, L. Cui, Z. Chang, A. Ma, T. Yin, R. Liang, Y. Han, Z. Wu, M. Zheng, C. Liu and L. Cai, Biomaterials, 2019, 214, 119226 CrossRef CAS PubMed.
- D. Huo, S. Liu, C. Zhang, J. He, Z. Zhou, H. Zhang and Y. Hu, ACS Nano, 2017, 11, 10159–10174 CrossRef CAS PubMed.
- C. Song, W. Xu, Z. Wei, C. Ou, J. Wu, J. Tong, Y. Cai, X. Dong and W. Han, J. Mater. Chem. B, 2020, 8, 648–654 RSC.
- D. Chen, Y. Tang, J. Zhu, J. Zhang, X. Song, W. Wang, J. Shao, W. Huang, P. Chen and X. Dong, Biomaterials, 2019, 221, 119422 CrossRef CAS PubMed.
- J. Nam, W.-G. La, S. Hwang, Y. S. Ha, N. Park, N. Won, S. Jung, S. H. Bhang, Y.-J. Ma, Y.-M. Cho, M. Jin, J. Han, J.-Y. Shin, E. K. Wang, S. G. Kim, S.-H. Cho, J. Yoo, B.-S. Kim and S. Kim, ACS Nano, 2013, 7, 3388–3402 CrossRef CAS PubMed.
- Z. Yu, M. Wang, W. Pan, H. Wang, N. Li and B. Tang, Chem. Sci., 2017, 8, 4896–4903 RSC.
- Y. Wang, K. Zhou, G. Huang, C. Hensley, X. Huang, X. Ma, T. Zhao, B. D. Sumer, R. J. DeBerardinis and J. Gao, Nat. Mater., 2014, 13, 204–212 CrossRef CAS PubMed.
- W. Huang, H. Zhao, J. Wan, Y. Zhou, Q. Xu, Y. Zhao, X. Yang and L. Gan, Theranostics, 2019, 9, 3825–3839 CrossRef CAS PubMed.
- Y. Tian, Y. Zhang, Z. Teng, W. Tian, S. Luo, X. Kong, X. Su, Y. Tang, S. Wang and G. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 2114–2122 CrossRef CAS PubMed.
- W. Deng, J. Qiu, S. Wang, Z. Yuan, Y. Jia, H. Tan, J. Lu and R. Zheng, Int. J. Nanomed., 2018, 13, 439–453 CrossRef CAS PubMed.
- Q. Wang, G. Sui, X. Wu, D. Teng, L. Zhu, S. Guan, H. Ran, Z. Wang and H. Wang, Acta Biomater., 2020, 102, 367–383 CrossRef CAS PubMed.
- Y.-L. Chen, F.-Q. Liu, Y. Guo, J. Cheng, L. Yang, M. Lu, P. Li, J. Xu, T. Yu, Z.-G. Wang, Y. Cao and H.-T. Ran, Biomater. Sci., 2018, 6, 2130–2143 RSC.
- X. Zhao, C.-X. Yang, L.-G. Chen and X.-P. Yan, Nat. Commun., 2017, 8, 14998 CrossRef CAS PubMed.
- X. Fang, C. Li, L. Zheng, F. Yang and T. Chen, Chem.–Asian J., 2018, 13, 996–1004 CrossRef CAS PubMed.
- M. R. Xie, Y. F. Zhu, S. B. Xu, G. W. Xu, R. Xiong, X. H. Sun and C. Q. Liu, Nanoscale, 2020, 12, 11497–11509 RSC.
- X. Deng, K. Li, X. Cai, B. Liu, Y. Wei, K. Deng, Z. Xie, Z. Wu, P. a. Ma, Z. Hou, Z. Cheng and J. Lin, Adv. Mater., 2017, 29, 1701266 CrossRef PubMed.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. S. Casalongue, V. Daniel and H. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- X. Sun, X. Huang, X. Yan, Y. Wang, J. Guo, O. Jacobson, D. Liu, L. P. Szajek, W. Zhu, G. Niu, D. O. Kiesewetter, S. Sun and X. Chen, ACS Nano, 2014, 8, 8438–8446 CrossRef CAS PubMed.
- Z. Zhou, Y. Wang, Y. Yan, Q. Zhang and Y. Cheng, ACS Nano, 2016, 10, 4863–4872 CrossRef CAS PubMed.
- X. Zheng, D. Xing, F. Zhou, B. Wu and W. R. Chen, Mol. Pharmaceutics, 2011, 8, 447–456 CrossRef CAS PubMed.
- Y. Wei, F. Zhou, D. Zhang, Q. Chen and D. Xing, Nanoscale, 2016, 8, 3530–3538 RSC.
- Z. Wang, R. Qiao, N. Tang, Z. Lu, H. Wang, Z. Zhang, X. Xue, Z. Huang, S. Zhang, G. Zhang and Y. Li, Biomaterials, 2017, 127, 25–35 CrossRef CAS PubMed.
- B. Li, Z. Jiang, D. Xie, Y. Wang and X. Lao, Int. J. Nanomed., 2018, 13, 7289–7302 CrossRef CAS PubMed.
- J. Liu, J. Zheng, H. Nie, D. Zhang, D. Cao, Z. Xing, B. Li and L. Jia, J. Colloid Interface Sci., 2019, 548, 131–144 CrossRef CAS PubMed.
- X. Zhao, Y. Huang, G. Yuan, K. Zuo, Y. Huang, J. Chen, J. Li and J. Xue, Chem. Commun., 2019, 55, 866–869 RSC.
- Z. Li, L. Zhu, W. Liu, Y. Zheng, X. Li, J. Ye, B. Li, H. Chen and Y. Gao, Acta Biomater., 2020, 107, 242–259 CrossRef CAS PubMed.
- C. Zhan, Y. Huang, C. Lin, S. Huang, F. Zeng and S. Wu, Small, 2019, 15, 1900309 CrossRef PubMed.
- V. Shanmugam, Y.-H. Chien, Y.-S. Cheng, T.-Y. Liu, C.-C. Huang, C.-H. Su, Y.-S. Chen, U. Kumar, H.-F. Hsu and C.-S. Yeh, ACS Appl. Mater. Interfaces, 2014, 6, 4382–4393 CrossRef CAS PubMed.
- C. Li, X.-Q. Yang, M.-Z. Zhang, Y.-Y. Song, K. Cheng, J. An, X.-S. Zhang, Y. Xuan, B. Liu and Y.-D. Zhao, Theranostics, 2018, 8, 5662–5675 CrossRef CAS PubMed.
- X. Ding, J. Liu, J. Li, F. Wang, Y. Wang, S. Song and H. Zhang, Chem. Sci., 2016, 7, 6695–6700 RSC.
- Y. Li, J. Lin, J. Ma, L. Song, H. Lin, B. Tang, D. Chen, G. Su, S. Ye, X. Zhu, F. Luo and Z. Hou, ACS Appl. Mater. Interfaces, 2017, 9, 34650–34665 CrossRef CAS PubMed.
- H. Wang, J. Chang, M. Shi, W. Pan, N. Li and B. Tang, Angew. Chem., Int. Ed., 2019, 58, 1057–1061 CrossRef CAS PubMed.
- Z. Zhang, Y. Wang, S. Xu, Y. Yu, A. Hussain, Y. Shen and S. Guo, J. Mater. Chem. B, 2017, 5, 5464–5472 RSC.
- H. Liu, T. Liu, X. Wu, L. Li, L. Tan, D. Chen and F. Tang, Adv. Mater., 2012, 24, 755–761 CrossRef CAS PubMed.
- J. L. Li, D. Day and M. Gu, Adv. Mater., 2008, 20, 3866 CrossRef CAS.
- H. S. Jung, W. H. Kong, D. K. Sung, M.-Y. Lee, S. E. Beack, D. H. Keum, K. S. Kim, S. H. Yun and S. K. Hahn, ACS Nano, 2014, 8, 260–268 CrossRef CAS PubMed.
- G. Mattheolabakis, L. Milane, A. Singh and M. M. Amiji, J. Drug Targeting, 2015, 23, 605–618 CrossRef CAS PubMed.
- L. Pan, J. Liu and J. Shi, ACS Appl. Mater. Interfaces, 2017, 9, 15952–15961 CrossRef CAS PubMed.
- H. Yuan, A. M. Fales and T. Vo-Dinh, J. Am. Chem. Soc., 2012, 134, 11358–11361 CrossRef CAS PubMed.
- W. Zhang, X. Ding, H. Cheng, C. Yin, J. Yan, Z. Mou, W. Wang, D. Cui, C. Fan and D. Sun, Theranostics, 2019, 9, 5610–5625 CrossRef CAS PubMed.
- P. Gao, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 6035–6071 RSC.
- H. S. Jung, J.-H. Lee, K. Kim, S. Koo, P. Verwilst, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2017, 139, 9972–9978 CrossRef CAS PubMed.
- H. Wang, J. Chang, W. Pan, N. Li and B. Tang, Nanoscale, 2019, 11, 18021–18025 RSC.
- Y. Shen, X. Zhang, L. Liang, J. Yue, D. Huang, W. Xu, W. Shi, C. Liang and S. Xu, Carbon, 2020, 156, 558–567 CrossRef CAS.
- K. Qiu, Y. Wen, C. Ouyang, X. Liao, C. Liu, T. W. Rees, Q. Zhang, L. Jib and H. Chao, Chem. Commun., 2019, 55, 11235–11238 RSC.
- D. Kand, L. Pizarro, I. Angel, A. Avni, D. Friedmann-Morvinski and R. Weinstain, Angew. Chem., Int. Ed., 2019, 58, 4659–4663 CrossRef CAS PubMed.
- W. Li, J. Yang, L. Luo, M. Jiang, B. Qin, H. Yin, C. Zhu, X. Yuan, J. Zhang, Z. Luo, Y. Du, Q. Li, Y. Lou, Y. Qiu and I. You, Nat. Commun., 2019, 10, 3349 CrossRef PubMed.
- J. Saleem, L. Wang and C. Chen, Adv. Healthcare Mater., 2018, 7, 1800525 CrossRef PubMed.
- L. A. Liotta and E. C. Kohn, Nature, 2001, 411, 375–379 CrossRef CAS PubMed.
- D. M. Gilkes, G. L. Semenza and D. Wirtz, Nat. Rev. Cancer, 2014, 14, 430–439 CrossRef CAS PubMed.
- A. K. Pedersen, J. M. L. de Melo, N. Morup, K. Tritsaris and S. F. Pedersen, BMC Cancer, 2017, 17, 542 CrossRef CAS PubMed.
- X. Ma, N. Gong, L. Zhong, J. Sun and X. J. Liang, Biomaterials, 2016, 97, 10–21 CrossRef CAS PubMed.
- K. Qiu, Y. Chen, T. W. Rees, L. Ji and H. Chao, Coord. Chem. Rev., 2019, 378, 66–86 CrossRef CAS.
- J. Zou, P. Wang, Y. Wang, G. Liu, Y. Zhang, Q. Zhang, J. Shao, W. Si, W. Huang and X. Dong, Chem. Sci., 2019, 10, 268–276 RSC.
- S. Chen, Q. Lei, W. X. Qiu, L. H. Liu, D. W. Zheng, J. X. Fan, L. Rong, Y. X. Sun and X. Z. Zhang, Biomaterials, 2017, 117, 92–104 CrossRef CAS PubMed.
- S. Chen, J. Fan, W. Qiu, F. Liu, G. Yan, X. Zeng and X. Zhang, J. Mater. Chem. B, 2018, 6, 1543–1551 RSC.
- Y. Shen, L. Liang, S. Zhang, D. Huang, R. Deng, J. Zhang, H. Qu, S. Xu, C. Liang and W. Xu, ACS Appl. Mater. Interfaces, 2018, 10, 7910–7918 CrossRef CAS PubMed.
- H. Zhou, H. Xu, X. Li, Y. Lv, T. Ma, S. Guo, Z. Huang, X. Wang and P. Xu, Mater. Sci. Eng., C, 2017, 81, 261–270 CrossRef CAS PubMed.
- W. Xu, J. Qian, G. Hou, A. Suo, Y. Wang, J. Wang, T. Sun, M. Yang, X. Wan and Y. Yao, ACS Appl. Mater. Interfaces, 2017, 9, 36533–36547 CrossRef CAS PubMed.
- J. Wang, H. Wang, L. Yan, Z. Hu, X. Wu and F. Li, RSC Adv., 2019, 9, 5270–5281 RSC.
- W. Xu, J. Qian, G. Hou, Y. Wang, J. Wang, T. Sun, L. Ji, A. Suo and Y. Yao, Acta Biomater., 2019, 83, 400–413 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. Li, J. Chen, C. Wang, M. Shao, S. T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 50, 7385–7390 CrossRef CAS PubMed.
- X. Nan, X. Zhang, Y. Liu, M. Zhou, X. Chen and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 9986–9995 CrossRef CAS PubMed.
- Y. Li, T. M. Duy Le, Q. Nam Bui, H. Y. Yang and D. S. Lee, Carbohydr. Polym., 2019, 226, 115281 CrossRef CAS PubMed.
- Y. Song, Q. Shi, C. Zhu, Y. Luo, Q. Lu, H. Li, R. Ye, D. Du and Y. Lin, Nanoscale, 2017, 9, 15813–15824 RSC.
- N. Li, Q. Sun, Z. Yu, X. Gao, W. Pan, X. Wan and B. Tang, ACS Nano, 2018, 12, 5197–5206 CrossRef CAS PubMed.
- Y. Cheng, F. Yang, K. Zhang, Y. Zhang, Y. Cao, C. Liu, H. Lu, H. Dong and X. Zhang, Adv. Funct. Mater., 2019, 29, 1903850 CrossRef CAS.
- H. Peng, J. Tang, R. Zheng, G. Guo, A. Dong, Y. Wang and W. Yang, Adv. Healthcare Mater., 2017, 6, 1601289 CrossRef PubMed.
- Y. Wang, R. Huang, G. Liang, Z. Zhang, P. Zhang, S. Yu and J. Kong, Small, 2014, 10, 109–116 CrossRef CAS PubMed.
- Y. J. Lu, P. Y. Lin, P. H. Huang, C. Y. Kuo, K. T. Shalumon, M. Y. Chen and J. P. Chen, Nanomaterials, 2018, 8, 193 CrossRef PubMed.
- M. Wang, C. You, Z. Gao, H. Wu, B. Sun, X. Zhu and R. Chen, J. Biomater. Sci., Polym. Ed., 2018, 29, 1360–1374 CrossRef CAS PubMed.
- T. S. Anilkumar, Y.-J. Lu, H.-A. Chen, H.-L. Hsu, G. Jose and J.-P. Chen, J. Magn. Magn. Mater., 2019, 473, 241–252 CrossRef CAS.
- H. Wu, C. You, J. Jiao, F. Chen, B. Sun and X. Zhu, Nanotechnology, 2019, 30, 3 Search PubMed.
- E. B. Kang, I. In, K.-D. Lee and S. Y. Park, J. Ind. Eng. Chem., 2017, 55, 224–233 CrossRef CAS.
- D. Yao, S. Yang, Y. Wang, K. Bian, W. Yang, D. Wang and B. Zhang, Nanoscale, 2019, 11, 6307–6314 RSC.
- Y. Wang, G. Wei, X. Zhang, X. Huang, J. Zhao, X. Guo and S. Zhou, Small, 2018, 14, 1702994 CrossRef PubMed.
- R. Guo, H. Peng, Y. Tian, S. Shen and W. Yang, Small, 2016, 12, 4541–4552 CrossRef CAS PubMed.
- S. H. Kim, I. In and S. Y. Park, Biomacromolecules, 2017, 18, 1825–1835 CrossRef CAS PubMed.
- Q. Li, J. Yang, Y. Chen, X. Zhou, D. Chen, Y. Li and X. Zhu, Mol. Pharm., 2018, 15, 4049–4062 CrossRef CAS PubMed.
- X. Cai, A. Bandla, C. K. Chuan, G. Magarajah, L.-D. Liao, D. B. L. Teh, B. K. Kennedy, N. V. Thakor and B. Liu, Mater. Horiz., 2019, 6, 311–317 RSC.
- B. Du, X. Cao, F. Zhao, X. Su, Y. Wang, X. Yan, S. Jia, J. Zhou and H. Yao, J. Mater. Chem. B, 2016, 4, 2038–2050 RSC.
- Y. Cao, T. Wu, K. Zhang, X. Meng, W. Dai, D. Wang, H. Dong and X. Zhang, ACS Nano, 2019, 13, 1499–1510 CAS.
- D. Chen, J. Zhang, Y. Tang, X. Huang, J. Shao, W. Si, J. Ji, Q. Zhang, W. Huang and X. Dong, J. Mater. Chem. B, 2018, 6, 4522–4530 RSC.
- L. Jing, H. Qu, D. Wu, C. Zhu, Y. Yang, X. Jin, J. Zheng, X. Shi, X. Yan and Y. Wang, Theranostics, 2018, 8, 2683–2695 CrossRef CAS.
- F. Chen, H. Hong, S. Goel, S. A. Graves, H. Orbay, E. B. Ehlerding, S. Shi, C. P. Theuer, R. J. Nickles and W. Cai, ACS Nano, 2015, 9, 3926–3934 CrossRef CAS PubMed.
- J. C. Yang, Y. Shang, Y. H. Li, Y. Cui and X. B. Yin, Chem. Sci., 2018, 9, 7210–7217 RSC.
- W. Gao, Y. Sun, M. Cai, Y. Zhao, W. Cao, Z. Liu, G. Cui and B. Tang, Nat. Commun., 2018, 9, 231 CrossRef PubMed.
- D. Wang, H. Dong, M. Li, X. Meng, Y. Cao, K. Zhang, W. Dai, C. Wang and X. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 6786–6794 CrossRef CAS.
- Z. Hussain, M. Arooj, A. Malik, F. Hussain, H. Safdar, S. Khan, M. Sohail, M. Pandey, H. Choudhury and H. Ei Thu, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1015–1024 CrossRef CAS PubMed.
- X. Zhen, J. Zhang, J. Huang, C. Xie, Q. Miao and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 7804–7808 CrossRef CAS PubMed.
- Y. Lyu, J. Zeng, Y. Jiang, X. Zhen, T. Wang, S. Qiu, X. Lou, M. Gao and K. Pu, ACS Nano, 2018, 12, 1801–1810 CrossRef CAS PubMed.
- J. Li, D. Cui, Y. Jiang, J. Huang, P. Cheng and K. Pu, Adv. Mater., 2019, 31, 1905091 CrossRef CAS PubMed.
- X. Zhen, C. Xie and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 3938–3942 CrossRef CAS PubMed.
- J. Li, J. Rao and K. Pu, Biomaterials, 2018, 155, 217–235 CrossRef CAS PubMed.
- J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38–71 RSC.
- X. Zhen, C. Xie, Y. Jiang, X. Ai, B. Xing and K. Pu, Nano Lett., 2018, 18, 1498–1505 CrossRef CAS PubMed.
- Y. Jiang, X. Zhao, J. Huang, J. Li, P. K. Upputuri, H. Sun, X. Han, M. Pramanik, Y. Miao, H. Duan, K. Pu and R. Zhang, Nat. Commun., 2020, 11, 1857 CrossRef CAS PubMed.
- Y. Jiang, P. K. Upputuri, C. Xie, Z. Zeng, A. Sharma, X. Zhen, J. Li, J. Huang, M. Pramanik and K. Pu, Adv. Mater., 2019, 31, 1808166 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |