Open Access Article
Open Access ArticleA FRET-based fluorescent Zn2+ sensor: 3D ratiometric imaging, flow cytometric tracking and cisplatin-induced Zn2+ fluctuation monitoring†
Hongxia
Xu‡
a,
Chengcheng
Zhu‡
a,
Yuncong
Chen
 *ab,
Yang
Bai
a,
Zhong
Han
a,
Shankun
Yao
a,
Yang
Jiao
a,
Hao
Yuan
a,
Weijiang
He
*ab,
Yang
Bai
a,
Zhong
Han
a,
Shankun
Yao
a,
Yang
Jiao
a,
Hao
Yuan
a,
Weijiang
He
 *a and
Zijian
Guo
*a and
Zijian
Guo
 *ab
*ab
aState Key Laboratory of Coordination Chemistry, Coordination Chemistry Institute, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, P. R. China. E-mail: chenyc@nju.edu.cn; heweij69@nju.edu.cn; zguo@nju.edu.cn
bChemistry and Biomedicine Innovation Center, Nanjing University, Nanjing 210023, P. R. China
First published on 15th September 2020
Abstract
Monitoring labile Zn2+ homeostasis is of great importance for the study of physiological functions of Zn2+ in biological systems. Here we report a novel ratiometric fluorescent Zn2+ sensor, CPBT, which was constructed based on chelation-induced alteration of FRET efficiency. CPBT was readily cell membrane permeable and showed a slight preferential localization in the endoplasmic reticulum. With this sensor, 3D ratiometric Zn2+ imaging was first realized in the head of zebra fish larvae via Z-stack mode. CPBT could track labile Zn2+ in a large number of cells through ratiometric flow cytometric assay. More interestingly, both ratiometric fluorescence imaging and flow cytometric assay demonstrated that the labile Zn2+ level in MCF-7 cells (cisplatin-sensitive) decreased while that in SKOV3 cells (cisplatin-insensitive) increased after cisplatin treatment, indicating that Zn2+ may play an important role in cisplatin induced signaling pathways in these cancer cells.
Introduction
Biological Zn2+ is an essential component for many proteins and is involved in various physiological processes such as gene transcription, neurotransmission, DNA repair, cell proliferation, apoptosis and redox responses.1–5 Disorder in Zn2+ homeostasis is associated with neurodegenerative diseases, cancer and immune defects.6–11 As a powerful technology which can provide spatiotemporal information on Zn2+, fluorescence imaging has been applied in living cells, tissues and model animals.12–16 Imaging and monitoring labile Zn2+ fluctuation is intriguing yet challenging since the deviation of endogenous labile Zn2+ homeostasis might be very small and dynamic. Moreover, interference from photobleaching, sensor concentration variation, and light scattering may result in misleading information. Therefore, ratiometric Zn2+ imaging is more reliable for accurate Zn2+ determination due to the self-calibration effect of dual emission.17–20Much effort has been devoted to ratiometric Zn2+ sensors,21–27 especially small molecule based sensors. Most of them were reported to image exogenous Zn2+ loaded into cells, and sensors displaying the ability to image endogenous, labile Zn2+ are still highly demanded. Developing ratiometric Zn2+ sensors with large emission shifts (>60 nm) and a minimum overlap between two emission bands is challenging yet desirable to improve the detection accuracy and sensitivity. In addition, 3D ratiometric imaging could provide more information on spatial distribution of Zn2+, which is appealing and requires sensors with near infrared (NIR) or two-photon excited fluorescence (TPEF) to improve the tissue penetration depth. Although fluorescence imaging is an ideal technique to provide detailed information on Zn2+ distribution in a single cell, it can only observe a limited number of cells in the field of view. In this regard, flow cytometry stands out as an attractive fluorescence technique to monitor Zn2+ fluctuations in a large number of cells.28–30
Cisplatin, which covalently binds with DNA to induce DNA damage, is one of the most widely used platinum-based antitumor drugs in clinics. Its DNA-adduct has been proposed to trigger pathways such as ATR, p53, p73, and MAPK to induce tumor cell apoptosis.31 Zn2+ has been proposed to be involved in many of these pathways, including the p53 pathway.10,32,33 Zinc deficiency may not only induce an increase in oxidative stress that causes DNA damage but also affect the expression of DNA-repair protein apurinic endonuclease (APE) and the downstream signaling events.9,34 We envisioned that intracellular Zn2+ homeostasis would be disturbed by cisplatin treatment, and different Zn2+ responses may be related to the sensitivity of cancer cells to cisplatin. Therefore, temporal monitoring of cisplatin-triggered labile Zn2+ fluctuations in different cancer cells is of great significance for the study of Zn2+ physiology during cisplatin-induced DNA damage, which may shed some light on the future development of new anti-cancer drugs.
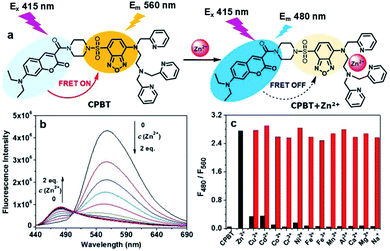
Herein, we report a novel FRET-based ratiometric Zn2+ sensor, CPBT, which is constructed by integrating a two-photon excitable coumarin derivative with an intramolecular charge transfer (ICT) fluorophore 4-amine-7-sulfamoylbenzo[c][1,2,5]-oxadiazole (ASBD). The electron donating group (4-amino group) of the ASBD fluorophore was modified with the Zn2+ ionophore N,N,N′-tri(pyridin-2-ylmethyl)ethane-1,2-diamine (TPEA), and Zn2+ binding would decrease the electron-donating ability of the 4-amino group and weaken the ICT effect in the ASBD fluorophore. A distinct blue shift of ASBD absorption would be observed due to Zn2+-chelation-induced alteration of the ICT effect.24,35 We envisioned that the large blue shift would diminish the spectral-overlap between donor emission and acceptor absorption, leading to a significant decrease in the FRET efficiency of CPBT (Fig. 1a). CPBT showed a specific Zn2+-induced large emission blue shift of ∼80 nm. Besides 2D ratiometric imaging, 3D ratiometric mapping of endogenous labile Zn2+ distribution in the head of zebrafish larvae was realized using CPBT. In addition, detection of endogenous labile Zn2+ in a large number of cells was achieved by ratiometric flow cytometry. More interestingly, both ratiometric fluorescence imaging and flow cytometric assay demonstrated an opposite change of labile Zn2+ in cisplatin-sensitive MCF-7 (human breast cancer cell line, Zn2+ decrease) and cisplatin-insensitive SKOV3 (human ovarian cancer cell line, Zn2+ increase) after cisplatin treatment. Considering the involvement of Zn2+ in cisplatin-related cell apoptosis pathways such as p53 and DNA damage repair, the current results might offer new clues to clarify the antitumor mechanism of cisplatin and the related drug resistance.
Results and discussion
Synthesis and characterization
CPBT was synthesized from coumarin-2-carboxylic acid in a 4-step procedure (Scheme S1†) and fully characterized by 1H NMR, 13C NMR and HRMS (see ESI†). Compounds 2 and 3 were prepared as the analogues of the donor and acceptor fluorophores in CPBT for comparison.Spectroscopic study and Zn2+-sensing behaviour of CPBT
The distinct overlap between donor (coumarin) emission and acceptor (ASBD) absorption suggested a high FRET efficiency of CPBT, which was confirmed by the strong emission at 560 nm and weak emission at 480 nm (Fig. 1b, S4a and b†). After Zn2+ binding, the absorption spectra of the ASBD moiety showed a large blue shift due to the decreased ICT effect (Fig. S4b and c†), leading to a dramatic decrease in the spectral-overlap and FRET efficiency. As a result, with the increasing concentration of Zn2+, the emission intensity at 560 nm significantly decreased and that at 480 nm increased distinctly. The intensity ratio of F480/F560 increased with the decrease of FRET efficiency. Two emission bands are well separated, which is beneficial for larger enhancement of the emission ratio and more accurate Zn2+ sensing. The absorption titration assay suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between CPBT and Zn2+ (Fig. S4d†). As shown in Fig. 1c, Zn2+ treatment induced a large fluorescence intensity ratio enhancement from 0.053 to 2.76, while other metal ions showed negligible effect on the emission ratio. Only small enhancements are induced by biologically scarce metals Cd2+ and Cu2+, which will not cause significant interference with Zn2+ sensing in biological samples. Moreover, the ratiometric Zn2+ sensing ability was intact in the presence of other metal ions. The results indicated that CPBT showed highly specific ratiometric Zn2+ sensing ability. In addition, the emission ratios of F480/F560 for both the free sensor and Zn2+-bound CPBT were stable in the pH range from 4.5 to 8.0, suggesting that the Zn2+ sensing behavior of CPBT could function well in the physiological pH range (Fig. S5†). The Kd value of the Zn2+/CPBT complex was determined to be 14 pM in a competitive binding experiment (Fig. S6†), indicating the high sensitivity of CPBT to Zn2+. The two-photon absorption cross-section is over 40 GM at 780–830 nm (Fig. S7†), indicating that CPBT might be suitable for two-photon excited fluorescence imaging.
1 stoichiometry between CPBT and Zn2+ (Fig. S4d†). As shown in Fig. 1c, Zn2+ treatment induced a large fluorescence intensity ratio enhancement from 0.053 to 2.76, while other metal ions showed negligible effect on the emission ratio. Only small enhancements are induced by biologically scarce metals Cd2+ and Cu2+, which will not cause significant interference with Zn2+ sensing in biological samples. Moreover, the ratiometric Zn2+ sensing ability was intact in the presence of other metal ions. The results indicated that CPBT showed highly specific ratiometric Zn2+ sensing ability. In addition, the emission ratios of F480/F560 for both the free sensor and Zn2+-bound CPBT were stable in the pH range from 4.5 to 8.0, suggesting that the Zn2+ sensing behavior of CPBT could function well in the physiological pH range (Fig. S5†). The Kd value of the Zn2+/CPBT complex was determined to be 14 pM in a competitive binding experiment (Fig. S6†), indicating the high sensitivity of CPBT to Zn2+. The two-photon absorption cross-section is over 40 GM at 780–830 nm (Fig. S7†), indicating that CPBT might be suitable for two-photon excited fluorescence imaging.
2D fluorescence imaging of Zn2+ in cells and zebrafish larvae
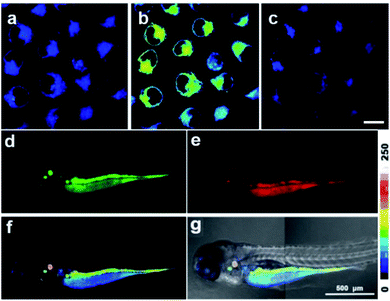
The desirable ratiometric Zn2+ sensing performance in a buffered solution encouraged us to evaluate its Zn2+ imaging ability in biological samples. 2D ratiometric Zn2+ imaging in living cells was conducted using fluorescence laser scanning confocal microscopy (LSCM). CPBT was readily cell membrane permeable and a co-localization assay showed that CPBT preferentially localized in the endoplasmic reticulum (Fig. S8†). As shown in Fig. 2a–c and S9†, CPBT stained MCF-7 cells displayed a dark-blue ratiometric image, which turned to a green-yellow color after exogenous Zn2+ treatment and back to a dark-blue color after incubation with Zn2+ scavenger N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN). The results indicated that the level of labile Zn2+ in the cytosol could be elevated by Zn2+ incubation and decreased by TPEN treatment. In addition, the chelatable [Zn2+] content in the cytoplasm of MCF-7 cells was estimated to be around 4.2 pM (Fig. 2a and S9j†). CPBT exhibits similar intracellular distribution patterns and ratiometric Zn2+ imaging performance in other cell lines, such as A549, SGC-7901, and SKOV3 cells, indicating that this sensor is a suitable candidate to monitor fluctuations in labile Zn2+ levels in live cells (Fig. S10 and S11†). Next, the in vivo ratiometric mapping of endogenous labile Zn2+ in zebrafish larvae was performed by LSCM (Fig. 2d–g). 3 day-old larvae were incubated with CPBT (0.1 μM) for 12 h. The fluorescence image of the red channel showed that CPBT dispersed mostly in the abdomen and parts of the head and trunk, while the images of the green channel showed only limited bright regions. The colors from blue to light red in the ratiometric channel correspond to different levels of endogenous labile Zn2+. The highest labile Zn2+ level was observed around the ears, similar to literature reports showing that uniquely high concentrations of synaptic Zn2+ were found in the dorsal cochlear nucleus of rats.36–383D fluorescence imaging of Zn2+
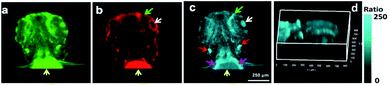
The 2D confocal imaging only shows the Zn2+ distribution in a specific focal plane, which is insufficient to disclose the overall spatial fluctuations in Zn2+ levels in living systems.39,40 Therefore, in vivo 3D ratiometric imaging of Zn2+ levels in the head of zebrafish larvae was performed for the first time using Z-stack mode with an effective imaging depth of ∼150 μm (Fig. S12†). Different distribution patterns of the 3D fluorescence and ratiometric images are observed (Fig. 3a–d). Both the top and side views of the 3D ratiometric image indicate that the highest level of labile Zn2+ is observed in the ears (red arrows in Fig. 3c) and the upper front superficial region of the yolk sac (magenta arrows in Fig. 3c). Moreover, the specific distribution pattern of labile Zn2+ in the eyeball demonstrated that the central pupil with a higher Zn2+ level (white arrows in Fig. 3c) is encircled by a dark ring representing a low Zn2+ level. The exterior represents the periphery and bottom of the eyeball, which are interspersed with a few regions with high Zn2+ levels that form an exterior ring (green arrow in Fig. 3c). These observations suggest that Zn2+ may play important roles in auditory and visual signal transduction. The results confirmed that compared with 2D ratiometric imaging, 3D ratiometric imaging could provide much more spatial information on the labile Zn2+ distribution in biological samples.Ratiometric flow cytometric assay for labile Zn2+ in live cells
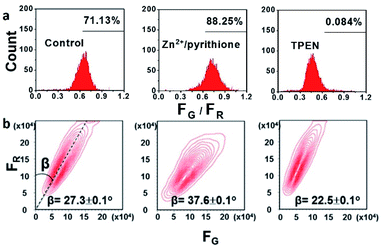
Flow cytometry is an invasive fluorescence technique to monitor Zn2+ fluctuations in a large number of cells, while fluorescence imaging can only observe a limited number of cells. However, the reports for ratiometric flow cytometric assay of Zn2+ levels are rare. Based on the successful ratiometric Zn2+ imaging with CPBT, we adopted a ratiometric flow cytometry protocol through CPBT staining to track the intracellular Zn2+ fluctuations in a large number of cells (Fig. 4 and S13†). The dual channel flow cytometric mode with a green channel of 450 ± 25 nm and a red channel of 570 ± 15 nm was adopted to determine the Zn2+-bound sensor (FG) and free sensor (FR) emission in each cell. The average FG/FR ratio of CPBT stained SKOV3 cells increased from 0.72 to 0.75 after Zn2+ treatment and then decreased to 0.46 upon TPEN treatment (Fig. 4a).To monitor the Zn2+ fluctuation in a more intuitive manner, we proposed a novel cell population contour plot method. The contour plot for the cell population is readily produced from the double-staining flow cytometry assay by treating the green and red channel as the emissions from the Zn2+-bound form and free sensor, respectively. A straight line crosses all the contour lines and forms an angle (β) with the longitudinal coordinate, and the tangent of the angle β is exactly the FG/FR ratio. Therefore, the fluctuation of labile Zn2+ levels could be estimated by the change of the β angle. The β angle of SKOV3 cells increases from 27.3 ± 0.1° to 37.6 ± 0.1° by Zn2+ treatment and decreases to 22.5 ± 0.1° upon TPEN treatment (Fig. 4b). Thus, ratiometric flow cytometry using CPBT staining is an effective technique for determining the intracellular labile Zn2+ levels and the β angle analysis of the cell number contour map in FG–FR coordinates is a feasible method for estimating the Zn2+ levels in different batches of cells.
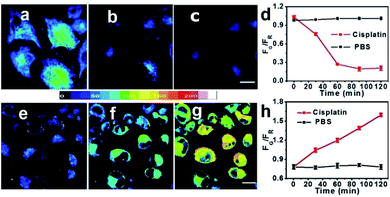
Tracking of endogenous labile Zn2+ fluctuation in cisplatin-treated MCF-7 and SKOV3 cells
Cisplatin is one of the most widely used clinical antitumor drugs to induce DNA damage to cancer cells, while Zn2+ has been proposed to be associated with different pathways related to DNA damage repair. Therefore, we try to evaluate whether CPBT is able to monitor the fluctuation of endogenous labile Zn2+ induced by cisplatin-stimuli. Ratiometric imaging assay was conducted first, and the CPBT-stained (10 μM, 1 h, 25 °C) cells were treated with cisplatin (20 μM, 25 °C) containing PBS (Fig. 5 and S14–17†). For the MCF-7 cells, the average ratio profile (FG/FR) indicated that the labile Zn2+ level decreased in the initial 60 min upon cisplatin treatment and remained at a low level thereafter (Fig. 5a–d). However, the labile Zn2+ level in SKOV3 cells showed a gradual enhancement at a stable rate in the initial 2 h of cisplatin incubation (Fig. 5e–h). Similar trends were also observed using commercial available Zn2+ sensor FluoZin-3 (Fig. S18 and S19†). The ratiometric response could be reversed by addition of Zn2+ for MCF-7 cells and pretreatment with TPEN for SKOV3 cells (Fig. S20†). Flow cytometry assay was conducted using a cell culture medium, and labile Zn2+ decrease in MCF-7 cells and increase in SKOV3 cells upon cisplatin treatment were observed with an obvious delay in the response time (Fig. S21–S27†). The β angle of MCF-7 cells suggested a steady decrease in the labile Zn2+ level in the initial 8 h of cisplatin incubation, while SKOV3 cells exhibited a gradual increase in labile Zn2+ levels during the cisplatin incubation of 10 h. The distinct difference of labile Zn2+ change in MCF-7 and SKOV3 cells induced by cisplatin incubation might be correlated with their different sensitivities to cisplatin. MCF-7 cells are cisplatin sensitive with an IC50 value of 5.7 ± 0.2 μM, while SKOV3 cells are cisplatin insensitive with an IC50 value of 42.5 ± 0.7 μM. The cell toxicity of CPBT was also evaluated, the result demonstrated that over 90% of the MCF-7 cells remain viable even for 40 μM CPBT incubation (Fig. S28†). The results suggested that high levels of labile Zn2+ might be beneficial for cisplatin resistance, which is in accordance with reports showing that Zn2+ is involved in the DNA damage repair pathway.34,41Conclusions
In summary, we have developed a ratiometric sensor for labile Zn2+ based on the Zn2+-induced ‘on–off’ switch of the FRET process. CPBT showed a good separation (80 nm) of the two emission bands, high specificity and affinity to Zn2+. Besides the 2D ratiometric Zn2+ imaging in living cells and the zebrafish, 3D ratiometric mapping of endogenous labile Zn2+ distribution in the head of zebrafish larvae was realized. In addition, a novel ratiometric flow cytometry assay was successfully applied for tracking endogenous labile Zn2+. More importantly, the cisplatin induced labile Zn2+ change was monitored using CPBT. Both ratiometric fluorescence imaging and flow cytometric assay showed that the labile Zn2+ level in MCF-7 cells (cisplatin-sensitive) decreased while that in SKOV3 cells (cisplatin-insensitive) increased after cisplatin treatment, confirming that Zn2+ plays an important role in the DNA repair in cancer cells. Further in-depth investigation to explore the impact of cisplatin on labile Zn2+ fluctuation and the downstream signaling pathways to apoptosis is undergoing in our lab. This study not only provided a novel and reliable design strategy for the development of Zn2+ ratiometric sensors, but also offered new ratiometric protocols for monitoring labile Zn2+ homeostasis, which would be beneficial for further exploration of Zn2+ biology.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant no. 21977044, 21907050, 21731004 and 91953201), the Natural Science Foundation of Jiangsu Province (BK20190282), and the Excellent Research Program of Nanjing University (ZYJH004).Notes and references
- J. M. Berg and Y. Shi, Science, 1996, 271, 1081–1085 Search PubMed.
- T. V. O'Halloran, Science, 1993, 261, 715–725 Search PubMed.
- S. C. Burdette and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3605–3610 Search PubMed.
- P. I. Oteiza, Free Radical Biol. Med., 2012, 53, 1748–1759 Search PubMed.
- M. Murakami and T. Hirano, Cancer Sci., 2008, 99, 1515–1522 Search PubMed.
- C. J. Frederickson, J.-Y. Koh and A. I. Bush, Nat. Rev. Neurosci., 2005, 6, 449–462 Search PubMed.
- Y. Li, L. Andereggen, K. Yuki, K. Omura, Y. Yin, H. Y. Gilbert, B. Erdogan, M. S. Asdourian, C. Shrock, S. de Lima, U. P. Apfel, Y. Zhuo, M. Hershfinkel, S. J. Lippard, P. A. Rosenberg and L. Benowitz, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E209–E218 Search PubMed.
- S. N. Loh, Metallomics, 2010, 2, 442–449 Search PubMed.
- E. Ho, J. Nutr. Biochem., 2004, 15, 572–578 Search PubMed.
- J. J. Miller, A. Blanchet, C. Orvain, L. Nouchikian, Y. Reviriot, R. M. Clarke, D. Martelino, D. Wilson, C. Gaiddon and T. Storr, Chem. Sci., 2019, 10, 10802–10814 Search PubMed.
- L. Rink and H. Haase, Trends Immunol., 2007, 28, 1–4 Search PubMed.
- E. L. Que, R. Bleher, F. E. Duncan, B. Y. Kong, S. C. Gleber, S. Vogt, S. Chen, S. A. Garwin, A. R. Bayer, V. P. Dravid, T. K. Woodruff and T. V. O'Halloran, Nat. Chem., 2015, 7, 130–139 Search PubMed.
- J. M. Goldberg, F. Wang, C. D. Sessler, N. W. Vogler, D. Y. Zhang, W. H. Loucks, T. Tzounopoulos and S. J. Lippard, J. Am. Chem. Soc., 2018, 140, 2020–2023 Search PubMed.
- Y. Chen, Y. Bai, Z. Han, W. He and Z. Guo, Chem. Soc. Rev., 2015, 44, 4517–4546 Search PubMed.
- C. Du, S. Fu, X. Wang, A. C. Sedgwick, W. Zhen, M. Li, X. Li, J. Zhou, Z. Wang, H. Wang and J. L. Sessler, Chem. Sci., 2019, 10, 5699–5704 Search PubMed.
- L. Fang, G. Trigiante, R. Crespo-Otero, C. S. Hawes, M. P. Philpott, C. R. Jones and M. Watkinson, Chem. Sci., 2019, 10, 10881–10887 Search PubMed.
- Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568–1600 Search PubMed.
- G. Grynkiewicz, M. Poenie and R. Y. Tsien, J. Biol. Chem., 1985, 260, 3440–3450 Search PubMed.
- Z.-X. Han, X.-B. Zhang, Z. Li, Y.-J. Gong, X.-Y. Wu, Z. Jin, C.-M. He, L.-X. Jian, J. Zhang, G.-L. Shen and R.-Q. Yu, Anal. Chem., 2010, 82, 3108–3113 Search PubMed.
- K. Sreenath, Z. Yuan, J. R. Allen, M. W. Davidson and L. Zhu, Chem.–Eur. J., 2015, 21, 867–874 Search PubMed.
- K. Komatsu, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 13447–13454 Search PubMed.
- Y. Qin, P. J. Dittmer, J. G. Park, K. B. Jansen and A. E. Palmer, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 7351–7356 Search PubMed.
- J. L. Vinkenborg, T. J. Nicolson, E. A. Bellomo, M. S. Koay, G. A. Rutter and M. Merkx, Nat. Methods, 2009, 6, 737–740 Search PubMed.
- Z. Liu, C. Zhang, Y. Chen, W. He and Z. Guo, Chem. Commun., 2012, 48, 8365–8367 Search PubMed.
- C. J. Chang, J. Jaworski, E. M. Nolan, M. Sheng and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1129–1134 Search PubMed.
- Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601–610 Search PubMed.
- M. L. Zastrow, Z. Huang and S. J. Lippard, ACS Chem. Biol., 2020, 15, 396–406 Search PubMed.
- H. Haase, S. Hebel, G. Engelhardt and L. Rink, Anal. Biochem., 2006, 352, 222–230 Search PubMed.
- D. Berendji, V. Kolb-Bachofen, K. L. Meyer, O. Grapenthin, H. Weber, V. Wahn and K.-D. Kröncke, FEBS Lett., 1997, 405, 37–41 Search PubMed.
- B. L. Fiedler, S. Van Buskirk, K. P. Carter, Y. Qin, M. C. Carpenter, A. E. Palmer and R. Jimenez, Anal. Chem., 2017, 89, 711–719 Search PubMed.
- D. Wang and S. J. Lippard, Nat. Rev. Drug. Discovery, 2005, 4, 307–320 Search PubMed.
- N. Habel, Z. Hamidouche, I. Girault, A. Patiño-García, F. Lecanda, P. J. Marie and O. Fromigué, Cell Death Dis., 2013, 4, e874 Search PubMed.
- E. Ho, C. Courtemanche and B. N. Ames, J. Nutr., 2003, 133, 2543–2548 Search PubMed.
- E. Ho and B. N. Ames, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 16770–16775 Search PubMed.
- Z. Liu, C. Zhang, Y. Chen, F. Qian, Y. Bai, W. He and Z. Guo, Chem. Commun., 2014, 50, 1253–1255 Search PubMed.
- S. A. Kodirov, S. Takizawa, J. Joseph, E. R. Kandel, G. P. Shumyatsky and V. Y. Bolshakov, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15218–15223 Search PubMed.
- A. Riccio, Y. Li, J. Moon, K. S. Kim, K. S. Smith, U. Rudolph, S. Gapon, G. L. Yao, E. Tsvetkov, S. J. Rodig, A. Van't Veer, E. G. Meloni, W. A. Carlezon, V. Y. Bolshakov and D. E. Clapham, Cell, 2009, 137, 761–772 Search PubMed.
- S. R. Wegst-Uhrich, E. J. Mullin, D. L. Ding, S. Manohar, R. Salvi, D. S. Aga and J. A. Roth, BioMetals, 2015, 28, 187–196 Search PubMed.
- F. Qian, C. Zhang, Y. Zhang, W. He, X. Gao, P. Hu and Z. Guo, J. Am. Chem. Soc., 2009, 131, 1460–1468 Search PubMed.
- Z. Guo, G.-H. Kim, J. Yoon and I. Shin, Nat. Protoc., 2014, 9, 1245–1254 Search PubMed.
- Y. Song, S. W. Leonard, M. G. Traber and E. Ho, J. Nutr., 2009, 139, 1626–1631 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc03037f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |