Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic three-component C–C bond forming dearomatization of bromoarenes with malonates and diazo compounds†
Hiroki
Kato
a,
Itsuki
Musha
a,
Masaaki
Komatsuda
a,
Kei
Muto
 *b and
Junichiro
Yamaguchi
*b and
Junichiro
Yamaguchi
 *a
*a
aDepartment of Applied Chemistry, Waseda University, 3-4-1, Ohkubo, Shinjuku, Tokyo 169-8555, Japan. E-mail: junyamaguchi@waseda.jp
bWaseda Institute for Advanced Study, Waseda University, Shinjuku, Tokyo 169-8050, Japan. E-mail: keimuto@aoni.waseda.jp
First published on 29th July 2020
Abstract
A Pd-catalyzed dearomative three-component C–C bond formation of bromoarenes with diazo compounds and malonates was developed. Various bromoarenes ranging from benzenoids to azines and heteroles were transformed to the corresponding substituted alicyclic molecules. The key to this reaction is the generation of a benzyl–palladium intermediate, which reacts with malonates to form a Pd–O-enolate species. Strikingly, the present method enabled rapid access to multi-substituted alicycles through subsequent elaboration of dearomatized products.
Introduction
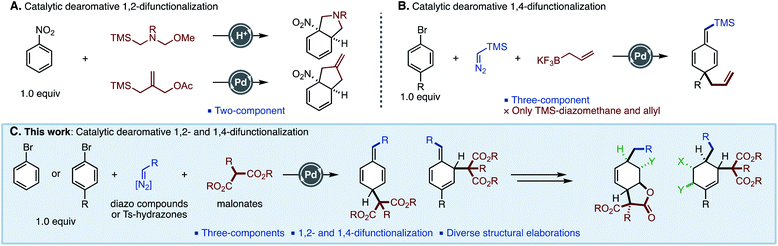
Dearomatization is a powerful method to generate molecular complexity, because this transformation can convert two-dimensional chemical feedstock arenes to highly valuable three-dimensional (alicyclic) architectures. Because of its potential, the development of dearomative methods is a topic of intense study in organic synthesis.1 Birch reduction2 and metal-catalyzed hydrogenations3 are the most established transformations, as well as oxidative dearomatizations. However, as a drawback, the vast majority of dearomative functionalizations rely on the electronic nature of the parent arenes.4 The dearomative reactions of non-activated arenes such as benzenes and naphthalenes have often seen a lack of reaction efficiency, requiring stoichiometric amounts of metal reagents and excess substrates.5 Although a few dearomative reactions of inactive arenes as a limiting agent (including catalytic fashion) have recently emerged, the development of dearomative methods of a new class of arenes is still highly valuable.6,7Dearomative difunctionalization of benzenoids is a useful and step-economical methodology to build three-dimensional carbon frameworks. As representative examples using benzenoids as a limiting reagent, photo-induced intramolecular cycloadditions,8 reactions of metal–arene complexes,9 and nucleophilic dearomatizations7,10 have been developed. Despite these advances, they still require the tedious preparation of metal–arene complexes and the presence of specific substituents such as oxazolines and iodanes. In this context, catalytic dearomative two-component C–C bond formations of nitroarenes with ylides were reported by Piettre and Trost, independently (Fig. 1A).11a,b These reactions enabled the dearomatization of one equivalent of nitroarenes. 1,2-Difunctionalized products were commonly obtained, and only one example of 1,4-difunctionalization was shown. Alternatively, we recently developed a Pd-catalyzed three-component dearomatization of bromoarenes by using TMS–diazomethane and allyl–BF3K, realizing two simultaneous C–C bond formations at 1,4-positions on the benzenoid (Fig. 1B).11c The key of this reaction was the generation of a Pd–benzyl complex intermediate through a Pd–carbene migratory insertion.12 However, applicable carbon units were limited to TMS–diazomethane and an allyl nucleophile, which hampered further synthetic applications of the method. In order to perform a dearomative synthesis of multi-functionalized carbocyclic molecules, the development of new methods to introduce other functional groups is needed. To this end, we envisaged that an ester functionality in the form of a malonate would be a versatile handle for further derivatization. However, it is known that malonates predominantly react with Pd–benzyl complexes to give benzyl substitution products.13,14 Although one report by the group of Bao showed a dearomative alkylation of benzyl chlorides with malonates, only naphthylmethyl chlorides were suitable substrates.15 Herein, we report the development of a Pd-catalyzed three-component C–C bond forming dearomatization of haloarenes using malonate nucleophiles, giving 1,4- and 1,2-difunctionalized alicyclic molecules. This method can use aryl N-tosylhydrazones16 as an alternative to TMS–diazomethane. Furthermore, it was found that the present methodology was amenable to arenes with various electronic nature, including azines and heteroles.
Results and discussion
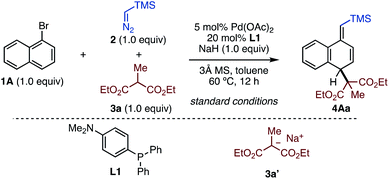
First, we explored optimized conditions for the dearomative alkylation using 1-bromonaphthalene (1A), TMS–diazomethane (2), and diethylmalonate 3a as model substrates (Table 1). Through extensive investigations, we identified the best conditions with Pd(OAc)2 (5.0 mol%) and L1 (20 mol%) as the catalyst, along with NaH, and 3 Å molecular sieves (MS) in toluene at 60 °C, obtaining desired dearomatized product 4Aa in 88% yield (entry 1, see the ESI† for details). Of all the triarylphosphines tested in this screening, decreasing the electron-donating ability of the phosphine led to a lower yield of 4Aa (entries 2–4). Regarding to diphosphines, DPEphos had a comparable effect to L1,producing 4Aa in a high yield (entry 5). The reaction in the absence of ligand generated no product (entry 6). The choice of base was critical: when less basic LiOtBu and Cs2CO3 were used instead of NaH, the yield of 4Aa significantly dropped (entries 7 and 8). The reaction in the absence of 3 Å MS decreased the yield of 4Aa (entry 9). It was found that preformed sodium enolate 3a′ was also an applicable alkylating agent under the reaction conditions without NaH (entry 10). Additionally, this reaction favored non-polar solvents, as cyclohexane also afforded 4Aa in 54% yield (entry 11). Through these studies, no benzylic substitution was observed.| Entry | Variation from standard conditions | 4Aa /% |
|---|---|---|
| a Conditions: 1A (0.20 mmol), 2 (0.20 mmol), 3a (0.20 mmol), Pd(OAc)2 (5.0 mol%), L1 (20 mol%), NaH (1.0 equiv.), 3 Å MS (50 mg), toluene (1.0 mL), 60 °C, 12 h. b NMR yield. c Without NaH and 3 Å MS at 70 °C. | ||
| 1 | None | 88 |
| 2 | P(p-anisyl)3 instead of L1 | 81 |
| 3 | PPh3 instead of L1 | 58 |
| 4 | P(C6F5)3 instead of L1 | 0 |
| 5 | DPEphos (10 mol%) instead of L1 | 92 |
| 6 | Without L1 | 0 |
| 7 | LiOtBu instead of NaH | 2 |
| 8 | Cs2CO3 instead of NaH | 0 |
| 9 | Without 3 Å MS | 71 |
| 10 | 3a′ was used instead of 3ac | 62 |
| 11 | Cyclohexane instead of toluene | 54 |
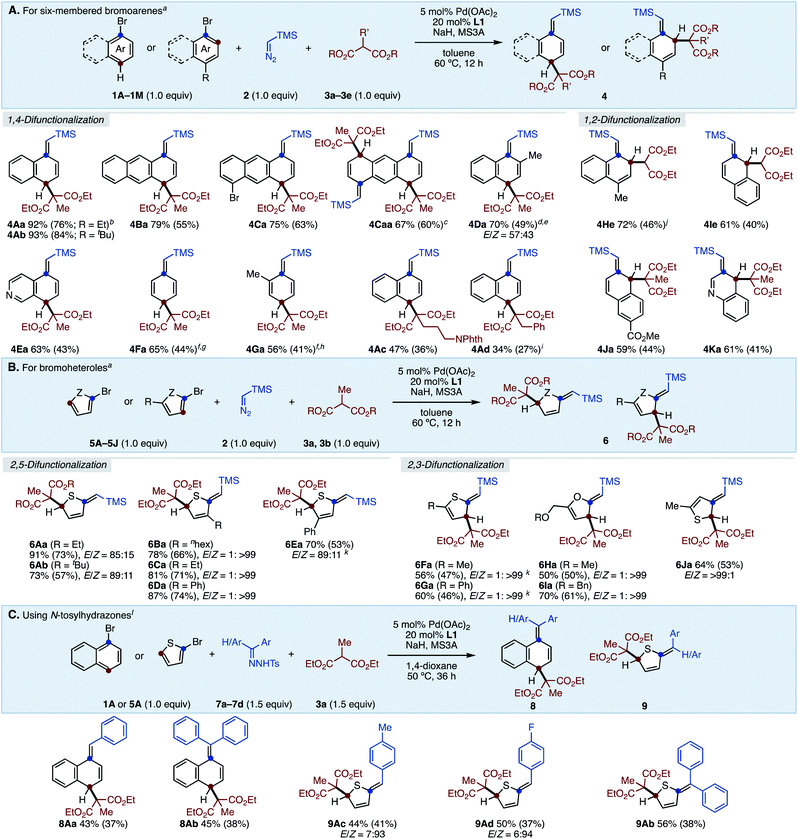
With the optimized conditions in hand, we next investigated the substrate generality of this reaction (Scheme 1A). As π-extended aromatics, anthracene also showed good reactivity to deliver dihydroanthracene 4Ba in high yield. The reaction of dibromoanthracene 1C smoothly proceeded to give dearomatized product 4Ca in good yield by using one equivalent of malonate 3a. For the same substrate 1C, increasing the amounts of 2, 3a, and NaH to two equivalents each furnished tetrahydroanthracene 4Caa in 67% NMR yield. Bromoarene with steric hindrance around the C–Br bond was also applicable to this reaction, generating C2-methyl-substituted product 4Da in good yield albeit as a diastereoisomeric mixture (57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43). 5-Bromoisoquinoline (1E) was successfully dearomatized under the present conditions, giving 4Ea in moderate yield. It is noteworthy that less reactive and simple phenyl rings were successfully converted to the corresponding cyclohexadiene skeletons 4Fa and 4Ga (see the ESI† for details). Although rearomatization occurred during the reactions probably due to the low stability of the products, 4Fa and 4Ga can be isolated in 44% and 41% yields, respectively. Regarding the scope of malonates, it was found that the nature of the ester groups did not affect the reaction efficiency, yielding 4Ab in almost the same yield as 4Aa. Moreover, we subjected diethylmalonates bearing phthalimidylalkyl (3c) as well as benzyl (3d) to this protocol, generating the corresponding products 4Ac and 4Ad in moderate yields. Probably due to the steric hinderance around the active methine moiety, higher temperatures were required for the reaction of 3d. Under the present conditions, only malonates can be used and other active methylene compounds were ineffective (see the ESI† for details). On the other hand, in the case of “para-substituted” bromoarenes, the reaction occurred in a 1,2-difunctionalization fashion. 4-Methylbromonaphthalene (1H) as well as 2-bromonaphthalenes (1I and 1J) gave 1,2-difunctionalized compounds. Pleasingly, the dearomative three-component C–C bond formation of 4-bromoquinoline (1K) was successful, furnishing a 3,4-dihydroquinoline core in a good yield.
43). 5-Bromoisoquinoline (1E) was successfully dearomatized under the present conditions, giving 4Ea in moderate yield. It is noteworthy that less reactive and simple phenyl rings were successfully converted to the corresponding cyclohexadiene skeletons 4Fa and 4Ga (see the ESI† for details). Although rearomatization occurred during the reactions probably due to the low stability of the products, 4Fa and 4Ga can be isolated in 44% and 41% yields, respectively. Regarding the scope of malonates, it was found that the nature of the ester groups did not affect the reaction efficiency, yielding 4Ab in almost the same yield as 4Aa. Moreover, we subjected diethylmalonates bearing phthalimidylalkyl (3c) as well as benzyl (3d) to this protocol, generating the corresponding products 4Ac and 4Ad in moderate yields. Probably due to the steric hinderance around the active methine moiety, higher temperatures were required for the reaction of 3d. Under the present conditions, only malonates can be used and other active methylene compounds were ineffective (see the ESI† for details). On the other hand, in the case of “para-substituted” bromoarenes, the reaction occurred in a 1,2-difunctionalization fashion. 4-Methylbromonaphthalene (1H) as well as 2-bromonaphthalenes (1I and 1J) gave 1,2-difunctionalized compounds. Pleasingly, the dearomative three-component C–C bond formation of 4-bromoquinoline (1K) was successful, furnishing a 3,4-dihydroquinoline core in a good yield.
Bromoheteroles were also reactive under the present reaction system (Scheme 1B). 2-Bromothiophene (5A) dearomatively assembled with 2 and malonates 3 to afford the corresponding dihydrothiophenes 6Aa and 6Ab in good yields with moderate E-selectivity. C3-Substituted 2-bromothiophenes also smoothly underwent the dearomative reaction, giving 2,5-difunctionalized heterocycles (6Ba–6Da) with remarkable Z-selectivity due to the steric repulsion. 4-Phenyl-2-bromothiophene (5E) was converted to 6Ea in good yield. In contrast, bromoheteroles bearing substituents at the C5-position led to 2,3-difunctionalization, yielding the corresponding dearomatized products 6Fa–6Ia in moderate yield with exclusive Z-selectivity. 3-Bromothiophene was dearomatized in a 2,3-difunctionalization manner (6Ja).
Furthermore, although diazo esters were not applicable under these conditions (see the ESI† for details), we found that aryl N-tosylhydrazones16 can be used in this reaction instead of TMS–diazomethane (Scheme 1C). For instance, aryl N-tosylhydrazones successfully reacted with 1-bromonaphthalene (1A) as well as 2-bromothiophene (5A) to give the corresponding products in moderate yields. Dearomatized products with tetra-substituted olefins were also synthesized by using diphenyl N-tosylhydrazone 7b in acceptable yields.
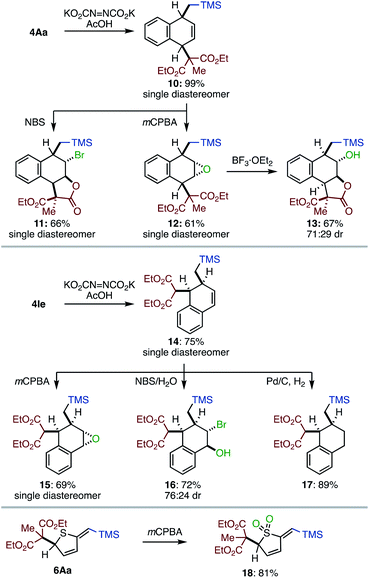
The most significant value of the method is that the dearomatized product can be elaborated to various multi-substituted alicyclic systems (Scheme 2). Partial reduction of 4Aa under diimide conditions produced dihydronaphthalene 10 in high yield with remarkable diastereoselectivity. The remaining olefin of 10 can be further functionalized. For instance, the treatment of 10 with NBS furnished bromolactone 11 in good yield with high diastereoselectivity. mCPBA oxidation of 10 produced epoxide 12 as a single diastereoisomer; epoxide 12 was further converted by using BF3·OEt2 to construct trans C–O bonds on the cyclic skeleton (13). A similar structural diversification was operative for 1,2-difunctionalized product 4Ie. Diastereo- and site-selective reduction of 4Ie, followed by epoxidation, bromohydrin formation, or hydrogenation furnished the corresponding multiply-functionalized alicyclic systems (15–17). Heterocycle 6Aa was successfully converted to sulfone 18 in good yield. These results strongly support that the present dearomative method can lead to a diverse range of highly functionalized alicyclic systems.
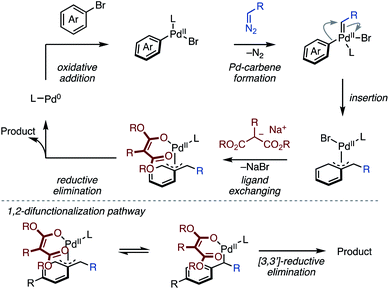
Our proposed mechanism is outlined in Scheme 3. The first oxidative addition of a bromoarene, followed by a Pd–carbene formation with a diazo compound generates an Ar–Pd–carbene complex. The Pd–carbene species leads to migratory insertion12 of the aryl moiety, giving a Pd–benzyl complex. Subsequently, the Pd–benzyl complex reacts with a sodium malonate to produce a benzyl–Pd–O-enolate species. The final C–C bond formation from the Pd–O-enolate species releases a dearomatized product. In this reaction mechanism, the key for the final C–C bond formation would be the generation and reactivity of the benzyl–Pd–O-enolate intermediate.17 The highly coordinating ability of malonates probably favors formation of the benzyl–Pd–O-enolates species, which then allows the inner-sphere C–C bond formation to give 1,4-difunctionalized products. Although a detailed mechanism and the site-selectivity17,18 is unclear at this stage, in the case of C4-substituted arenes, the C–C bond formation at the C2 position takes place from a σ-benzyl–Pd–O-enolate. This likely occurs a 3,3′-sigmatropic reductive elimination mechanism, probably due to steric repulsion between the malonate moiety and the C4-substituent.19
Conclusions
In summary, we have developed the dearomative three-component C–C bond formation of bromoarenes with diazo compounds and malonates by a palladium catalyst. The reaction was applicable to a variety of aryl bromides including azines and heteroles, which suggests that this reaction system is not restricted to the electronic nature of the arenes. The structural elaboration of the dearomatized products showcase the synthetic utility of the present method. Further studies to develop asymmetric dearomatizations using a chiral catalyst20 and to elucidate the reaction mechanism are underway in our laboratory.21Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP19H02726 (to J. Y.), JP20H04829 (hybrid catalysis), JP19K15573, and The SATOMI Scholarship Foundation (to K. M.). The Materials Characterization Central Laboratory in Waseda University is acknowledged for HRMS measurement.Notes and references
- S. P. Roche and J. A. Porco, Angew. Chem., Int. Ed., 2011, 50, 4068–4093 CrossRef CAS PubMed.
- For reviews, see: (a) A. J. Birch, Pure Appl. Chem., 1996, 68, 553–556 CAS; (b) A. G. Schultz, Chem. Commun., 1999, 1263–1271 RSC; (c) H. E. Zimmerman, Acc. Chem. Res., 2011, 45, 164–170 CrossRef PubMedFor a recent example, see: (d) B. K. Peters, K. X. Rodriguez, S. H. Reisberg, S. B. Beil, D. P. Hickey, Y. Kawamata, M. Collins, J. Starr, L. Chen, S. Udyavara, K. Klunder, T. J. Gorey, S. L. Anderson, M. Neurock, S. D. Minteer and P. S. Baran, Science, 2019, 363, 838–845 CrossRef CAS PubMed.
- For recent examples of catalytic arene hydrogenation, see: (a) D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557–2590 CrossRef CAS PubMed; (b) M. P. Wiesenfeldt, Z. Nairoukh, W. Li and F. Glorius, Science, 2017, 357, 908–912 CrossRef PubMed; (c) M. P. Wiesenfeldt, T. Knecht, C. Schlepphorst and F. Glorius, Angew. Chem., Int. Ed., 2018, 57, 8297–8300 CrossRef PubMed; (d) Z. Nairoukh, M. Wollenburg, C. Schlepphorst, K. Bergander and F. Glorius, Nat. Chem., 2019, 11, 264–270 CrossRef; (e) M. Wollenburg, D. Moock and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 6549–6553 CrossRef PubMed.
- (a) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662–12686 CrossRef PubMed; (b) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807–4815 RSC; (c) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570–1580 RSC; (d) C. Zheng and S.-L. You, Chem, 2016, 1, 830–857 CrossRef.
- For a review, see: (a) W. C. Wertjes, E. H. Southgate and D. Sarlah, Chem. Soc. Rev., 2018, 47, 7996–8017 RSCFor representative examples, see: (b) E. H. Southgate, J. Pospech, J. Fu, D. R. Holycross and D. Sarlah, Nat. Chem., 2016, 8, 922–928 CrossRef PubMed; (c) M. Okumura, S. M. Nakamata Huynh, J. Pospech and D. Sarlah, Angew. Chem., Int. Ed., 2016, 55, 15910–15914 CrossRef; (d) M. Okumura, A. S. Shved and D. Sarlah, J. Am. Chem. Soc., 2017, 139, 17787–17790 CrossRef CAS PubMed; (e) L. W. Hernandez, U. Klöckner, J. Pospech, L. Hauss and D. Sarlah, J. Am. Chem. Soc., 2018, 140, 4503–4507 CrossRef PubMed; (f) W. C. Wertjes, M. Okumura and D. Sarlah, J. Am. Chem. Soc., 2019, 141, 163–167 CrossRef PubMed; (g) C. Tang, M. Okumura, Y. Zhu, A. Hooper, Y. Lee and D. Sarlah, Angew. Chem., Int. Ed., 2019, 58, 10245–10249 CrossRef; (h) C. Tang, M. Okumura, H. Deng and D. Sarlah, Angew. Chem., Int. Ed., 2019, 58, 15762–15766 CrossRef PubMed; (i) K. L. Smith, C. L. Padgett, W. D. Mackay and J. S. Johnson, J. Am. Chem. Soc., 2020, 142, 6449–6455 CrossRef.
- For catalytic dearomative reactions using inactive arenes as a limiting reagent, see: (a) M. Bao, H. Nakamura and Y. Yamamoto, J. Am. Chem. Soc., 2001, 123, 759–760 CrossRef PubMed; (b) M. Komatsuda, K. Muto and J. Yamaguchi, Org. Lett., 2018, 20, 4354–4357 CrossRef PubMed; (c) Z.-P. Yang, R. Jiang, Q.-F. Wu, L. Huang, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2018, 57, 16190–16193 CrossRef PubMed; (d) A. Yanagimoto, M. Komatsuda, K. Muto and J. Yamaguchi, Org. Lett., 2020, 22, 3423–3427 CrossRef PubMed; (e) Y. Kayashima, M. Komatsuda, K. Muto and J. Yamaguchi, Chem. Lett., 2020, 49, 836 CrossRef.
- For non-catalytic reactions using non-activated arenes as a limiting reagent, see: (a) W. Zhao, X. Huang, Y. Zhan, Q. Zhang, D. Li, Y. Zhang, L. Kong and B. Peng, Angew. Chem., Int. Ed., 2019, 58, 17210–17214 CrossRef PubMed; (b) X. Huang, Y. Zhang, W. Liang, Q. Zhang, Y. Zhan, L. Kong and B. Peng, Chem. Sci., 2020, 11, 3048–3053 RSC.
- (a) R. Remy and C. G. Bochet, Chem. Rev., 2016, 116, 9816–9849 CrossRef PubMed; (b) B. K. Liebov and W. D. Harman, Chem. Rev., 2017, 117, 13721–13755 CrossRef PubMed.
- A. R. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917–2940 CrossRef PubMed.
- F. Lopez Ortiz, M. J. Iglesias, I. Fernandez, C. M. Andujar Sanchez and G. Ruiz Gomez, Chem. Rev., 2007, 107, 1580–1691 CrossRef PubMed.
- For examples of catalytic dearomative difunctionalization of one equivalent of benzenoids, see: (a) S. Lee, I. Chataigner and S. R. Piettre, Angew. Chem., Int. Ed., 2011, 50, 472–476 CrossRef PubMed; (b) B. M. Trost, V. Ehmke, B. M. O'Keefe and D. A. Bringley, J. Am. Chem. Soc., 2014, 136, 8213–8216 CrossRef PubMed; (c) M. Komatsuda, H. Kato, K. Muto and J. Yamaguchi, ACS Catal., 2019, 9, 8991–8995 CrossRef.
- For reviews and examples of Pd-carbene migratory insertion, see: (a) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810 CrossRef PubMed; (b) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef PubMed; (c) S. K. Devine and D. L. Van Vranken, Org. Lett., 2007, 9, 2047 CrossRef PubMed; (d) S. K. Devine and D. L. Van Vranken, Org. Lett., 2008, 10, 1909 CrossRef PubMed; (e) J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Adv. Synth. Catal., 2010, 352, 3235 CrossRef.
- (a) J.-Y. Legros and J.-C. Fiaud, Tetrahedron Lett., 1992, 33, 2509–2510 CrossRef; (b) J.-Y. Legros, M. Toffano and J.-C. Fiaud, Tetrahedron: Asymmetry, 1995, 6, 1899–1902 CrossRef CAS; (c) J.-Y. Legros, G. Primault, M. Toffano, M.-A. Rivière and J.-C. Fiaud, Org. Lett., 2000, 2, 433–436 CrossRef CAS PubMed; (d) R. Kuwano, Y. Kondo and Y. Matsuyama, J. Am. Chem. Soc., 2003, 125, 12104–12105 CrossRef CAS PubMed; (e) R. Kuwano and Y. Kondo, Org. Lett., 2004, 6, 3545–3547 CrossRef CAS.
- B. M. Trost and L. C. Czabaniuk, Angew. Chem., Int. Ed., 2014, 53, 2826–2851 CrossRef PubMed.
- B. Peng, S. Zhang, X. Yu, X. Feng and M. Bao, Org. Lett., 2011, 13, 5402–5405 CrossRef PubMed.
- For selected examples of Pd-catalyzed reaction of N-tosylhydrazones, see: (a) J. Barluenga, P. Moriel, C. Valdés and F. Aznar, Angew. Chem., Int. Ed., 2007, 46, 5587–5590 CrossRef PubMed; (b) L. Zhou, F. Ye, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2010, 132, 13590–13591 CrossRef PubMed; (c) J. Barluenga, M. Escribano, F. Aznar and C. Valdés, Angew. Chem., Int. Ed., 2010, 49, 6856–6859 CrossRef PubMed; (d) L. Zhou, F. Ye, J. Ma, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2011, 50, 3510–3514 CrossRef CAS PubMed; (e) J. Feng, B. Li, Y. He and Z. Gu, Angew. Chem., Int. Ed., 2016, 55, 2186–2190 CrossRef CAS; (f) K. Ishitobi, K. Muto and J. Yamaguchi, ACS Catal., 2019, 9, 11685–11690 CrossRef CAS.
- During the preparation of this manuscript, C–H alkylation involving a similar mechanism was reported, where a Pd-O-enolate benzyl complex was proposed as a key intermediate to construct a C–C bond at the C4 position. See: F. de Azambuja, M.-H. Yang, T. Feoktistova, M. Selvaraju, A. C. Brueckner, M. A. Grove, S. Koley, P. H.-Y. Cheong and R. A. Altman, Nat. Chem., 2020, 12, 489–496 CrossRef CAS PubMed.
- For the discussion on the site-selectivity in the case of allyl nucleophile, see: A. Ariafard and Z. Lin, J. Am. Chem. Soc., 2006, 128, 13010 CrossRef CAS.
- For selected Pd-catalyzed allylic alkylations involving an inner-sphere mechanism, see: (a) M. Meńdez, J. M. Cuerva, E. Goḿez-Bengoa, D. J. Caŕdenas and A. M. Echavarren, Chem.-Eur. J., 2002, 8, 3620–3628 CrossRef; (b) L. A. Brozek, M. J. Ardolino and J. P. Morken, J. Am. Chem. Soc., 2011, 133, 16778–16781 CrossRef CAS; (c) J. A. Keith, D. C. Behenna, N. Sherden, J. T. Mohr, S. Ma, S. C. Marinescu, R. J. Nielsen, J. Oxgaard, B. M. Stoltz and W. A. Goddard, J. Am. Chem. Soc., 2012, 134, 19050–19060 CrossRef CAS; (d) D.-C. Bai, F.-L. Yu, W.-Y. Wang, D. Chen, H. Li, Q.-R. Liu, C.-H. Ding, B. Chen and X.-L. Hou, Nat. Commun., 2016, 7, 11806–11816 CrossRef PubMed; (e) H.-C. Lin, P.-P. Xie, Z.-Y. Dai, S.-Q. Zhang, P.-S. Wang, Y.-G. Chen, T.-C. Wang, X. Hong and L.-Z. Gong, J. Am. Chem. Soc., 2019, 141, 5824–5834 CrossRef CAS.
- Preliminary results toward an asymmetric reaction are provided in the ESI.†.
- A prior version of the present article was deposited as a preprint on ChemRxiv: H. Kato, I. Musha, M. Komatsuda, K. Muto and J. Yamaguchi, ChemRxiv, 2020 DOI:10.26434/chemrxiv.12234740.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data for compounds including 1H and 13C NMR spectra. See DOI: 10.1039/d0sc02881a |
| This journal is © The Royal Society of Chemistry 2020 |