Open Access Article
Open Access ArticleGuest-responsive polaritons in a porous framework: chromophoric sponges in optical QED cavities†
Ritesh
Haldar
 *a,
Zhihua
Fu
a,
Reetu
Joseph
b,
David
Herrero
c,
Luis
Martín-Gomis
c,
Bryce S.
Richards
*a,
Zhihua
Fu
a,
Reetu
Joseph
b,
David
Herrero
c,
Luis
Martín-Gomis
c,
Bryce S.
Richards
 bd,
Ian. A.
Howard
bd,
Ian. A.
Howard
 bd,
Angela
Sastre-Santos
bd,
Angela
Sastre-Santos
 *c and
Christof
Wöll
*c and
Christof
Wöll
 *a
*a
aKarlsruhe Institute of Technology (KIT), Institute of Functional Interfaces (IFG), Hermann-von-Helmholtz Platz-1, Eggenstein-Leopoldshafen, 76344, Germany. E-mail: ritesh.haldar@kit.edu; christof.woell@kit.edu
bKarlsruhe Institute of Technology (KIT), Institute of Microstructure Technology (IMT), Hermann-von-Helmholtz Platz-1, Eggenstein-Leopoldshafen, 76344, Germany
cÁrea de Química Orgánica, Instituto de Bioingeniería, Universidad Miguel Hernández, Avda. de la Universidad, s/n, Elche 03202, Spain. E-mail: asastre@umh.es
dKarlsruhe Institute of Technology (KIT), Light Technology Institute (LTI), Engesserstrasse 13, Karlsruhe, 76131, Germany
First published on 13th July 2020
Abstract
Introducing porous material into optical cavities is a critical step toward the utilization of quantum-electrodynamical (QED) effects for advanced technologies, e.g. in the context of sensing. We demonstrate that crystalline, porous metal–organic frameworks (MOFs) are well suited for the fabrication of optical cavities. In going beyond functionalities offered by other materials, they allow for the reversible loading and release of guest species into and out of optical resonators. For an all-metal mirror-based Fabry–Perot cavity we yield strong coupling (∼21% Rabi splitting). This value is remarkably large, considering that the high porosity of the framework reduces the density of optically active moieties relative to the corresponding bulk structure by ∼60%. Such a strong response of a porous chromophoric scaffold could only be realized by employing silicon-phthalocyanine (SiPc) dyes designed to undergo strong J-aggregation when assembled into a MOF. Integration of the SiPc MOF as active component into the optical microcavity was realized by employing a layer-by-layer method. The new functionality opens up the possibility to reversibly and continuously tune QED devices and to use them as optical sensors.
Introduction
The engineering of the electronic structure of atoms and molecules by strong interactions with light inside optical resonators creates exciting prospects for materials science.1–3 The strong coupling of optical and electronic states can give rise – often in counterintuitive ways – to new functionalities. In this emerging field, the new phenomena are not limited to the optical regime;4–6 in previous works it has been demonstrated that such quantum-electro-dynamical (QED) phenomena can affect many more molecular properties, including vibrational transitions, chemical reactivity landscapes, and electronic properties.7–9 When an organic or inorganic compound is introduced into an optical cavity (for example, by sandwiching it between two mirrors), electronic states of the molecular moieties can resonate with the eigenmodes of the cavity and give rise to two new polaritonic states, P+ and P− (Rabi splitting).The size of the Rabi splitting energy, Δ = P+ − P− depends on the energy of the optical transition energy, absorption coefficient, and orientation of the transition dipole relative to the cavity. Recent progress in the field of organic semiconductor (OSC) materials10 suggest that subtle tuning of the transition dipole moment orientation can significantly enhance Rabi splitting energies.10,11 Whereas adjusting the optical transition energies of an individual organic chromophoric moiety is rather straight forward (e.g. by attaching appropriate side groups), the optical properties of the corresponding condensed molecular solid are difficult to predict because of cooperative effects and since even slight modification of the molecular structure can result in a completely different packing.12,13
In the majority of previously reported organic materials yielding strong QED coupling cases,2 the optically active dyes were embedded in a random fashion into a polymer. As a result, the corresponding transition dipole moments are randomly oriented, a fact which carries substantial disadvantages with regard to a rational optimization of the couplings and a thorough theoretical analysis. For future device applications, crystalline cavity materials thus would be highly desirable. Indeed, crystalline materials have been used as active materials, including perovskites,14,15 and organic semiconductors.16 In the latter case, however, only few successful efforts were reported, e.g. for anthracene and rubrene.17,18
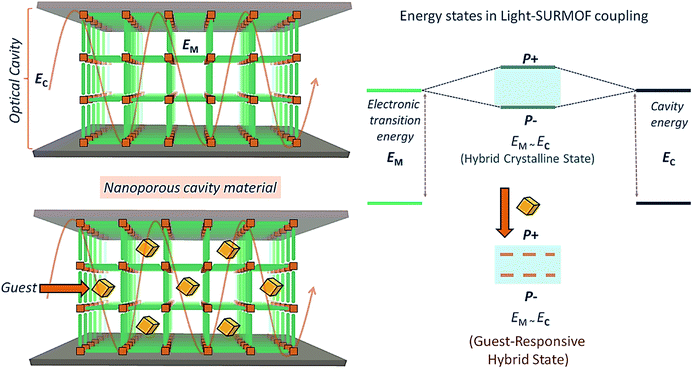
For further advancement of QED device applications, the reversibly loading and unloading of guest species would be of interest. The reversible loading and release of guest molecules (see Fig. 1) could be used to tune the permittivity and thus the optical response of the microresonator, thus adding new functionalities to these optical resonators. However, creating voids inside a materials composed of chromophores reduces their density and thus the overall optical response. As a result, there have been no previous efforts along this direction.
Here, we demonstrate that metal–organic frameworks (MOFs)19,20 based on highly active, J-aggregate21 forming chromophoric linkers in connection with layer-by-layer (lbl) deposition methods offer the possibility to fabricate well-defined, crystalline arrays of dye molecules to serve as porous cavity materials in optical resonators. Our demonstration devices were built using simple and easy-to-fabricate top and bottom metal mirrors. In the past, MOFs have not been considered good candidates to be used as active materials in microcavities because the voids enclosed by these molecular frameworks reduces the density of optically active chromophores. The intrinsic porosity of these crystalline coordination networks allows to reversibly load guest species into the porous MOFs at high densities (Fig. 1). This particular property immediately makes new applications possible, e.g. in the fields of optoelectronics, sensing, and catalysis. Finally, the lbl fabrication method together with highly optically active chromophoric MOFs allows fabrication of “simple” cavities defined by a top and bottom mirror – a substantial simplification as compared to the multi-step fabrication of distributed Bragg reflector (DBR) mirrors.
MOFs are intrinsically crystalline, porous materials fabricated by assembling building units of at least two types, metal or metal/oxo connectors and organic ligands, into periodic structures.19,20 Large building block libraries are available, and the number of already characterized materials exceeds 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. With regard to the design of optically active assemblies, MOFs built from chromophoric linkers are of particular interest.22 The list of modified photoactive organic compounds suitable as MOF linkers already has an impressive length, and the variation of optical transition energies and absorption cross-sections by chemical modifications of the basic chromophore is rather straightforward. Moreover, attaching such additional functionalities can be accomplished without changes of crystal structure, allowing for rational crystal engineering approaches.23 MOFs have already been successfully fabricated from many organic dyes, including porphyrins,24 perylene/naphthalenediimide25,26 or phthalocyanines.27 In addition, the knowledge of exact spatial geometry and possibility of isoreticular chemistry (i.e. construction of identical lattice topology by different chromophores) make the MOF materials ideally suited as an active cavity material.
000. With regard to the design of optically active assemblies, MOFs built from chromophoric linkers are of particular interest.22 The list of modified photoactive organic compounds suitable as MOF linkers already has an impressive length, and the variation of optical transition energies and absorption cross-sections by chemical modifications of the basic chromophore is rather straightforward. Moreover, attaching such additional functionalities can be accomplished without changes of crystal structure, allowing for rational crystal engineering approaches.23 MOFs have already been successfully fabricated from many organic dyes, including porphyrins,24 perylene/naphthalenediimide25,26 or phthalocyanines.27 In addition, the knowledge of exact spatial geometry and possibility of isoreticular chemistry (i.e. construction of identical lattice topology by different chromophores) make the MOF materials ideally suited as an active cavity material.
The common powder form of MOFs, synthesized using solvothermal methods, however, is not well suited for optical applications.22 Here, we demonstrate that so-called SURMOFs, surface-anchored MOF (SURMOF) monolithic thin films constructed by lbl method,28 are well suited to fabricate Fabry–Perot-type optical resonators showing strong exciton–photon coupling. This first demonstration of a MOF-based optical cavity showing a large Rabi-splitting is based on a particularly optically active silicon phthalocyanine (SiPc 1) linker. The strong photon–matter coupling is enhanced by the fact that assembling the SiPc linkers into a MOF yields a J-aggregate, increasing its oscillator strength. Fabrication of the cavity with the lbl technique is straightforward. The key step is the deposition of a SURMOF on a modified Ag-substrate, the bottom mirror, which is then coated with Ag to yield the top mirror (Ag deposition uses PVD). Note, that in previous cases the fabrication of microcavities from crystalline OSC materials with strong coupling required the fabrication of DBR mirrors.17,18 Although DBR mirrors exhibit huge quality factors (Q factors), they carry certain disadvantages. First, a considerable effort in manufacturing is needed. Second for such cavities the effective optical path, which interacts with the material is reduced, and thus reducing the overall efficiency.29 Therefore, we here use the simpler metal-mirror approach. The effects of a smaller Q-factor are – at least in part – compensated by an enhancement of the photonic field caused by the presence of the metal mirrors11 and the fact that all of the optical path is located inside the active medium.29
The optical transition energy (EM) of the SiPc-based SURMOF was tuned in resonance to the optical cavity (EC) mode (Fig. 1) by adjusting the SURMOF thickness, which can be done conveniently by adjusting the number of deposition layers. We demonstrate an optical cavity with ultra-strong coupling, with a Rabi splitting energy of ∼21% of the parent optical transition energy of the SURMOF. In going beyond previous works, the porous nature of the mirror separator allows further tuning of the coupling by loading with guest species. In the following, we will present the design-principle of integrating nanoporous, crystalline SURMOFs into optical cavities, and discuss the prospects of this novel approach for sensing application.
Results and discussion
J-aggregates of SiPc in Zn-SURMOF-2
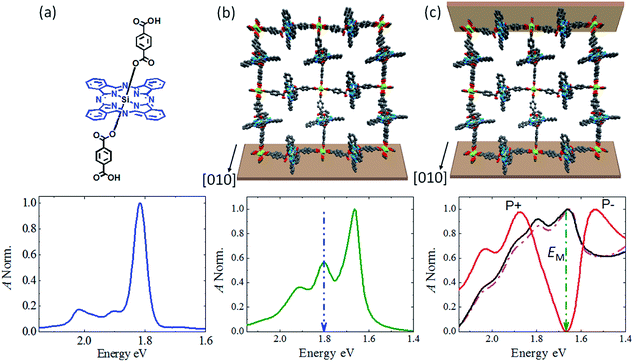
The realization of a MOF-based microcavity is based on a particularly well-suited class of MOFs, known as Zn-SURMOF-2,30 which is constructed by linking Zn-based paddle-wheel-type secondary building units (SBU) with ditopic carboxylate functionalized linkers to yield planes with quadratic subunits. These planes are then stacked to yield a 3D structure, containing 1D narrow channels running along the stacking direction, as shown in Fig. 2b and S1.† As the photoactive linker, we have chosen a silicon phthalocyanine (SiPc) (Fig. 2a). Pcs are particularly attractive dyes, because of their stability and pronounced tunability.31 In a recent work using a Zn(II) metallated Pc assembled in a polymer matrix,32 a clear splitting of the two Rabi-states could not be seen (less than 52 meV), a fact which is likely due to the amorphous nature of the previous cavity separators.Crystalline Pc materials have, not yet been used as active material in optical cavities most likely due to the difficulties in obtaining well-defined thin layers of these compounds, to overcome these limitations we have fabricated a Pc-based MOF linker by actually substituting an SiPc compound with 1,4-benzenedicarboxylic acid appends,33 see Fig. 2a (SiPc 1 linker synthesis described in ESI†). Using a lbl spin coating method, SiPc based crystalline SURMOFs Zn-SiPc were then grown on glass and self-assembled monolayer (SAM)-modified Ag substrates. Characterization by XRD (both, out-of-plane and in-plane) revealed a structure with lattice dimensions a = b = 2.1 nm, c = 1.1 nm (Fig. S2†). The [001] crystallographic direction is oriented perpendicular to the substrate surface, and the 1D pore channels running parallel to the surface plane (i.e. the [010] direction) (Fig. S1†). As shown in Fig. S1,† as a result of the fairly small interlayer distance of ∼1.1 nm the SiPc linkers of the neighboring 2D layers are in close contact and bring about substantial electronic interaction.34,35
In a first set of experiments, the optical properties of the Zn-SiPc SURMOF-2 grown on the SAM-modified Ag substrate were examined by UV-Vis spectroscopy. Fig. 2a and b reveals that the pronounced maximum at 1.82 eV observed for the solvated linker is substantially red-shifted, to 1.67 eV, after assembly into the SURMOF-2 structure. This pronounced shift provides strong indication for the formation of a J-aggregate,36 which is consistent with the head-to-tail arrangement of the SiPc transition dipole moments. This conclusion is further supported by the emission spectra (Fig. S3†), which reveal a substantially lower emission energy for the SURMOF (1.53 eV) than for the solvated linkers (1.7 eV). The formation of a J-aggregate, which has been reported for chromophoric MOFs only in a few cases23,37 supports strong light–matter coupling by enhancing the transition dipole moment.
SURMOF in optical cavity
In a next step, we have deposited Zn-SiPc SURMOFs of various thicknesses on SAM-modified Ag substrates, simply by using different numbers of immersion cycles. In following previous approaches,6,38–40 this thin film was converted into an optical cavity by coating with a ∼10 nm semi-transparent Ag top layer (Fig. 1a) using a metal evaporator. The quality (Q) factor of such a metal-mirror cavity is fairly low (∼10–100,2,41 compared to 103 to 105∼ for DBR cavities), but the high optical activity of the chromophoric active material still allows to achieve impressive optical performance, as demonstrated by the UV-Vis spectra shown in Fig. 2. Data recorded in transmission (T) and in reflectance (R) for different SURMOF-based cavities reveal strong resonance effects varying with SURMOF thickness, as shown in Fig. 2c.For thicknesses of 210 and 300 nm the absorbance (A = 1 − R − T)1 (black and pink lines, respectively) only reveals a subtle broadening of the absorption band. This is in accord with expectation, for such low thicknesses the cavity energy will be located at energies larger than 2 eV, way above the Zn-SiPc SURMOF excitation energy (1.67 eV). Increasing the number of deposition cycles to yield SURMOF thickness to 400 nm results in a distinct splitting of the absorbance band (red). Clearly, now the cavity state is in resonance with the optical Zn-SiPc transition, yielding a pronounced splitting of the absorption band into two new states located at 1.52 and 1.87 eV (marked as P+ and P−). The nearly complete quenching of the parent optical transition (green arrow) suggests that a majority of the SiPc molecules are in resonance with the cavity mode. The splitting energy of 0.35 eV amounts to ∼21% of the Zn-SiPc SURMOF-2 optical transition energy and reveals that the SURMOF cavity is in the regime of strong coupling.
This value is the largest so far reported for planar aromatic chromophores (porphyrin: 4%,42 phthalocyanine: ∼3% (ref. 32)) used as photoactive moieties in microcavities (see Table S1†). Higher values were only reported for nonplanar, more complex compounds, e.g. conjugated polymers (∼65%)10 and photoswitches (∼32%).43,44
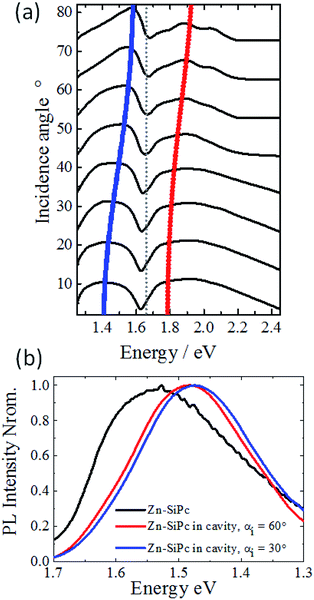
The presence of strong coupling in the SURMOF-based microcavity is further supported by the outcome of experiments where absorption spectra were recorded for different angles of incidence (Fig. 3a). The dispersive nature of the photonic component in the hybrid photon–matter state is evident from the energy versus incident angle (incident angle is related to the in-plane momentum as following: k‖ = 2π/λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ; θ = incident angle, λ = peak energy, Fig. 3a). With increasing momentum or incident angle, P+ state shows an increasing photonic component (red line) and P− state approaches to be more like a material (blue line), and these features resemble to the previously studied cases of strong Rabi splitting in vacuum field. We have also investigated the effect of varying the angle of incident light on the emission spectra. We found that the presence of strong coupling caused the emission spectrum of the SURMOF to shift to lower energy (1.48 eV at 3.30 eV excitation with incidence angle of 60°, see ESI† for details of experimental set up), compared to the pristine SURMOF (1.53 eV), as illustrated in Fig. 3b. Assuming that Kasha's rule is valid for the polaritons, radiative decay should occur from the lowest lying excited state (the new P− state created under strong coupling field), the emission maximum should shift to lower energies than that in the pristine material.38 Change in the incidence angle to 30° further shifted the emission maximum by ∼10 meV, confirming the dispersive nature of the hybrid state.
θ; θ = incident angle, λ = peak energy, Fig. 3a). With increasing momentum or incident angle, P+ state shows an increasing photonic component (red line) and P− state approaches to be more like a material (blue line), and these features resemble to the previously studied cases of strong Rabi splitting in vacuum field. We have also investigated the effect of varying the angle of incident light on the emission spectra. We found that the presence of strong coupling caused the emission spectrum of the SURMOF to shift to lower energy (1.48 eV at 3.30 eV excitation with incidence angle of 60°, see ESI† for details of experimental set up), compared to the pristine SURMOF (1.53 eV), as illustrated in Fig. 3b. Assuming that Kasha's rule is valid for the polaritons, radiative decay should occur from the lowest lying excited state (the new P− state created under strong coupling field), the emission maximum should shift to lower energies than that in the pristine material.38 Change in the incidence angle to 30° further shifted the emission maximum by ∼10 meV, confirming the dispersive nature of the hybrid state.
Guest-responsive polariton
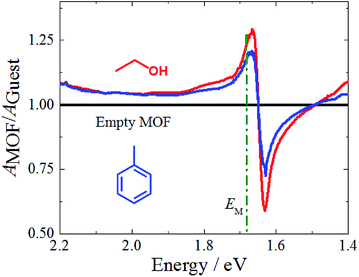
We now come to the demonstration of the unique properties of the new cavity material realized here. The MOF structure contains void space (confirmed by gravimetric vapor adsorption, Fig. S4†), and loading guest species having different refractive index (RI) into the pores of the framework material will change the effective permittivity of the cavity and thus the Rabi splitting. This is demonstrated by the data shown in Fig. 4, which reveal changes of the UV-Vis spectra upon loading with a prototype guest species, ethanol (RI = 1.36, Fig. S6†). For an incident angle of 60°, a pronounced 50 meV shift is observed for the P− state, much larger than the solvatochromic shifts observed for the Zn-SiPc SURMOFs immersed in ethanol, where the effect amounts to only 5 meV (Fig. S4†). Clearly, in the strong coupling regime, the guest-induced change of optical properties is strongly amplified. For other guest species, e.g. a nonpolar aromatic compound toluene (higher RI of 1.49 compared to that of ethanol), the effect was much smaller (∼20 meV), as expected (Fig. 3 and S6†).Conclusions
In conclusion, we have realized a new type of optical microcavity with a crystalline, highly oriented MOF as the active material. Despite the intrinsic porosity of this particular active material, the total amount of Rabi splitting achieved for the SiPc-based MOF is larger than observed before for conventional solid solutions of planar chromophores. The fabrication of the MOF-based cavity is simplified by the superior optical quality of the SURMOFs, which allows to use a simple metal-mirror approach; thus, avoiding the more complicated fabrication of DBR mirrors. The SiPc linkers used here yield a J-aggregate when assembled into a MOF material, one of the main reasons for the overall huge performance of the SURMOF microcavity. In this context, MOF materials offer a huge potential, since the arrangement of the chromophores inside the lattice can be tuned using steric control units,22 thus allowing further optimization of the optical response of these highly photoactive materials.By going beyond previous applications of optical cavities in the strong or ultra-strong coupling regime, the intrinsic porosity of the MOF material allowed for realization of a highly sensitive optical detector, where the common solvatochromic shift was found to be amplified by an order of magnitude. Such photon–matter hybrid state in porous materials provide an excellent platform for future advanced sensor applications. In addition, use of a MOF-based catalyst in a similar vacuum field to achieve vibrational strong coupling is another more attractive step to be considered in future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. H and C. W acknowledge support from Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—2082/1—390761711 (Cluster 3DMM2O) and SPP 1928 “COORNETS”.Notes and references
- T. W. Ebbesen, Hybrid Light–Matter States in a Molecular and Material Science Perspective, Acc. Chem. Res., 2016, 49, 2403–2412 CrossRef CAS PubMed.
- M. Hertzog, M. Wang, J. Mony and K. Börjesson, Strong light–matter interactions: a new direction within chemistry, Chem. Soc. Rev., 2019, 48, 937–961 RSC.
- A. Frisk Kockum, A. Miranowicz, S. De Liberato, S. Savasta and F. Nori, Ultrastrong coupling between light and matter, Nat. Rev. Phys., 2019, 1, 19–40 CrossRef.
- C. R. Gubbin, S. A. Maier and S. Kéna-Cohen, Low-voltage polariton electroluminescence from an ultrastrongly coupled organic light-emitting diode, Appl. Phys. Lett., 2014, 104, 233302 CrossRef.
- K. Georgiou, P. Michetti, L. Gai, M. Cavazzini, Z. Shen and D. G. Lidzey, Control over Energy Transfer between Fluorescent BODIPY Dyes in a Strongly Coupled Microcavity, ACS Photonics, 2018, 5, 258–266 CrossRef CAS.
- S. Takahashi, K. Watanabe and Y. Matsumoto, Singlet fission of amorphous rubrene modulated by polariton formation, J. Chem. Phys., 2019, 151, 074703 CrossRef PubMed.
- J. A. Hutchison, T. Schwartz, C. Genet, E. Devaux and T. W. Ebbesen, Modifying Chemical Landscapes by Coupling to Vacuum Fields, Angew. Chem., Int. Ed., 2012, 51, 1592–1596 CrossRef CAS PubMed.
- J. Lather, P. Bhatt, A. Thomas, T. W. Ebbesen and J. George, Cavity Catalysis by Cooperative Vibrational Strong Coupling of Reactant and Solvent Molecules, Angew. Chem., Int. Ed., 2019, 58, 10635–10638 CrossRef CAS PubMed.
- A. Thomas, L. Lethuillier-Karl, K. Nagarajan, R. M. A. Vergauwe, J. George, T. Chervy, A. Shalabney, E. Devaux, C. Genet, J. Moran and T. W. Ebbesen, Tilting a ground-state reactivity landscape by vibrational strong coupling, Science, 2019, 363, 615 CrossRef CAS PubMed.
- F. Le Roux, R. A. Taylor and D. D. C. Bradley, Enhanced and Polarization-Dependent Coupling for Photoaligned Liquid Crystalline Conjugated Polymer Microcavities, ACS Photonics, 2020, 7, 746–758 CrossRef CAS.
- S. Kéna-Cohen, S. A. Maier and D. D. C. Bradley, Ultrastrongly Coupled Exciton–Polaritons in Metal-Clad Organic Semiconductor Microcavities, Adv. Opt. Mater., 2013, 1, 827–833 CrossRef.
- L. Zhu, R. O. Al-Kaysi, R. J. Dillon, F. S. Tham and C. J. Bardeen, Crystal Structures and Photophysical Properties of 9-Anthracene Carboxylic Acid Derivatives for Photomechanical Applications, Cryst. Growth Des., 2011, 11, 4975–4983 CrossRef CAS.
- B. Zhang, H. Soleimaninejad, D. J. Jones, J. M. White, K. P. Ghiggino, T. A. Smith and W. W. H. Wong, Highly Fluorescent Molecularly Insulated Perylene Diimides: Effect of Concentration on Photophysical Properties, Chem. Mater., 2017, 29, 8395–8403 CrossRef CAS.
- W. Du, S. Zhang, J. Shi, J. Chen, Z. Wu, Y. Mi, Z. Liu, Y. Li, X. Sui, R. Wang, X. Qiu, T. Wu, Y. Xiao, Q. Zhang and X. liu, Strong Exciton–Photon Coupling and Lasing Behavior in All-Inorganic CsPbBr3 Micro/Nanowire Fabry-Pérot Cavity, ACS Photonics, 2018, 5, 2051–2059 CrossRef CAS.
- W. Du, S. Zhang, Q. Zhang and X. Liu, Recent Progress of Strong Exciton–Photon Coupling in Lead Halide Perovskites, Adv. Mater., 2019, 31, 1804894 CrossRef CAS PubMed.
- E. Orgiu, J. George, J. A. Hutchison, E. Devaux, J. F. Dayen, B. Doudin, F. Stellacci, C. Genet, J. Schachenmayer, C. Genes, G. Pupillo, P. Samorì and T. W. Ebbesen, Conductivity in organic semiconductors hybridized with the vacuum field, Nat. Mater., 2015, 14, 1123–1129 CrossRef CAS PubMed.
- S. Kéna-Cohen and S. R. Forrest, Room-temperature polariton lasing in an organic single-crystal microcavity, Nat. Photonics, 2010, 4, 371–375 CrossRef.
- Y. Tsuchimoto, H. Nagai, M. Amano, K. Bando and H. Kondo, Cavity polaritons in an organic single-crystalline rubrene microcavity, Appl. Phys. Lett., 2014, 104, 233307 CrossRef.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341, 1230444 CrossRef PubMed.
- N. J. Hestand and F. C. Spano, Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer, Chem. Rev., 2018, 118, 7069–7163 CrossRef CAS PubMed.
- R. Haldar, A. Mazel, M. Kristić, Q. Zhang, M. Jakoby, I. A. Howard, B. S. Richards, N. Jung, D. Jacquemin, S. Diring, W. Wenzel, F. Odobel and C. Wöll, A de novo strategy for predictive crystal engineering to tune excitonic coupling, Nat. Commun., 2019, 10, 2048 CrossRef PubMed.
- R. Haldar, L. Heinke and C. Wöll, Advanced Photoresponsive Materials Using the Metal–Organic Framework Approach, Adv. Mater., 2020, 32, 1905227 CrossRef CAS PubMed.
- S. Huh, S.-J. Kim and Y. Kim, Porphyrinic metal–organic frameworks from custom-designed porphyrins, CrystEngComm, 2016, 18, 345–368 RSC.
- E. Castaldelli, K. D. G. Imalka Jayawardena, D. C. Cox, G. J. Clarkson, R. I. Walton, L. Le-Quang, J. Chauvin, S. Ravi P Silva and G. J.-F. Demets, Electrical semiconduction modulated by light in a cobalt and naphthalene diimide metal-organic framework, Nat. Commun., 2017, 8, 2139 CrossRef PubMed.
- B. Lü, Y. Chen, P. Li, B. Wang, K. Müllen and M. Yin, Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion, Nat. Commun., 2019, 10, 767 CrossRef PubMed.
- H. Zhong, K. H. Ly, M. Wang, Y. Krupskaya, X. Han, J. Zhang, V. Kateav, B. Büchner, I. M. Weidinger, S. Kaskel, P. Liu, M. Chen, R. Dong and X. Feng, A Phthalocyanine-Based Layered Two-Dimensional Conjugated Metal–Organic Framework as a Highly Efficient Electrocatalyst for the Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2019, 58, 10677–10682 CrossRef CAS PubMed.
- J. Liu and C. Wöll, Surface-supported metal–organic framework thin films: fabrication methods, applications, and challenges, Chem. Soc. Rev., 2017, 46, 5730–5770 RSC.
- P. A. Hobson and W. L. Barnes, Strong exciton–photon coupling in a low-Q all-metal mirror microcavity, Appl. Phys. Lett., 2002, 81, 3519–3521 CrossRef CAS.
- J. Liu, B. Lukose, O. Shekhah, H. K. Arslan, P. Weidler, H. Gliemann, S. Bräse, S. Grosjean, A. Godt, X. Feng, K. Müllen, I.-B. Magdau, T. Heine and C. Wöll, A novel series of isoreticular metal organic frameworks: realizing metastable structures by liquid phase epitaxy, Sci. Rep., 2012, 2, 921 CrossRef PubMed.
- G. de la Torre, C. G. Claessens and T. Torres, Phthalocyanines: old dyes, new materials. Putting color in nanotechnology, Chem. Commun., 2007, 2000–2015 RSC.
- R. Jayaprakash, K. Georgiou, H. Coulthard, A. Askitopoulos, S. K. Rajendran, D. M. Coles, A. J. Musser, J. Clark, I. D. W. Samuel, G. A. Turnbull, P. G. Lagoudakis and D. G. Lidzey, A hybrid organic–inorganic polariton LED, Light Sci. Appl., 2019, 8, 81 CrossRef PubMed.
- L. Martín-Gomisa, E. M. Barea, F. Fernández-Lázaroa, J. Bisquert and A. Sastre-Santos, Dye sensitized solar cells using non-aggregated silicon phthalocyanines, J. Porphyr. Phthalocyanines, 2011, 15, 1004–1010 CrossRef.
- R. Haldar, A. Mazel, R. Joseph, M. Adams, I. A. Howard, B. S. Richards, M. Tsotsalas, E. Redel, S. Diring, F. Odobel and C. Wöll, Excitonically Coupled States in Crystalline Coordination Networks, Chem.–Eur. J., 2017, 23, 14316–14322 CrossRef CAS PubMed.
- R. Haldar, K. Batra, S. Marschner, A. B. Kuc, S. Zahn, R. A. Fischer, S. Bräse, T. Heine and C. Wöll, Bridging the Green Gap: Metal–Organic Framework Heteromultilayers Assembled from Porphyrinic Linkers Identified by Using Computational Screening, Chem.–Eur. J., 2019, 25, 7847–7851 CrossRef CAS PubMed.
- T. Doane, A. Chomas, S. Srinivasan and C. Burda, Observation and Photophysical Characterization of Silicon Phthalocyanine J-Aggregate Dimers in Aqueous Solutions, Chem.–Eur. J., 2014, 20, 8030–8039 CrossRef CAS PubMed.
- N. Sikdar, D. Dutta, R. Haldar, T. Ray, A. Hazra, A. J. Bhattacharyya and T. K. Maji, Coordination-Driven Fluorescent J-Aggregates in a Perylenetetracarboxylate-Based MOF: Permanent Porosity and Proton Conductivity, J. Phys. Chem. C, 2016, 120, 13622–13629 CrossRef CAS.
- X. Zhong, T. Chervy, L. Zhang, A. Thomas, J. George, C. Genet, J. A. Hutchison and T. W. Ebbesen, Energy Transfer between Spatially Separated Entangled Molecules, Angew. Chem., Int. Ed., 2017, 56, 9034–9038 CrossRef CAS PubMed.
- D. M. Coles, N. Somaschi, P. Michetti, C. Clark, P. G. Lagoudakis, P. G. Savvidis and D. G. Lidzey, Polariton-mediated energy transfer between organic dyes in a strongly coupled optical microcavity, Nat. Mater., 2014, 13, 712–719 CrossRef CAS PubMed.
- C.-Y. Cheng, R. Dhanker, C. L. Gray, S. Mukhopadhyay, E. R. Kennehan, J. B. Asbury, A. Sokolov and N. C. Giebink, Charged Polaron Polaritons in an Organic Semiconductor Microcavity, Phys. Rev. Lett., 2018, 120, 017402 CrossRef CAS PubMed.
- H. T. Miyazaki and Y. Kurokawa, Squeezing Visible Light Waves into a 3-nm-Thick and 55-nm-Long Plasmon Cavity, Phys. Rev. Lett., 2006, 96, 097401 CrossRef PubMed.
- D. G. Lidzey, D. D. C. Bradley, M. S. Skolnick, T. Virgili, S. Walker and D. M. Whittaker, Strong exciton–photon coupling in an organic semiconductor microcavity, Nature, 1998, 395, 53–55 CrossRef CAS.
- T. Schwartz, J. A. Hutchison, C. Genet and T. W. Ebbesen, Reversible Switching of Ultrastrong Light-Molecule Coupling, Phys. Rev. Lett., 2011, 106, 196405 CrossRef CAS PubMed.
- F. Barachati, J. Simon, Y. A. Getmanenko, S. Barlow, S. R. Marder and S. Kena-Cohen, Tunable Third-Harmonic Generation from Polaritons in the Ultrastrong Coupling Regime, ACS Photonics, 2018, 5, 119–125 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of SiPc, details of SURMOF and optical cavity fabrication and characterization. See DOI: 10.1039/d0sc02436h |
| This journal is © The Royal Society of Chemistry 2020 |