Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regioselective molybdenum-catalyzed allylic substitution of tertiary allylic electrophiles: methodology development and applications†
Muhammad
Salman‡
,
Yaoyao
Xu‡
,
Shahid
Khan
,
Junjie
Zhang
and
Ajmal
Khan
 *
*
Department of Applied Chemistry, School of Science, Xi'an Key Laboratory of Sustainable Energy Materials Chemistry, Xi'an Jiao Tong University, Xi'an 710049, P. R. China. E-mail: ajmalkhan@xjtu.edu.cn
First published on 6th May 2020
Abstract
The first molybdenum-catalyzed allylic sulfonylation of tertiary allylic electrophiles is described. The method employs a readily accessible catalyst (Mo(CO)6/2,2′-bipyridine, both are commercially available) and represents the first example of the use of a group 6 transition metal-catalyst for allylic sulfonylation of substituted tertiary allylic electrophiles to form carbon–sulfur bonds. This atom economic and operationally simple methodology is characterized by its relatively mild conditions, wide substrate scope, and excellent regioselectivity profile, thus unlocking a new platform to forge sulfone moieties, even in the context of late-stage functionalization and providing ample opportunities for further derivatization through traditional Suzuki cross-coupling reactions.
Introduction
The concept of the π-allyl metal-complex was first formulated by Tsuji in 1965 (ref. 1a) and, later, properly adopted by Trost in 1973.1b Since then, this technology has enabled organic chemists to create a host of novel procedures for the synthesis of simple to complex molecules.2 Among these is the development and utilization of heteroatom nucleophile reagents, such as oxygen, nitrogen, and sulfur-based nucleophiles.2,3 Despite the massive development that has been made in this area, there still remain untapped opportunities in the potential application of these heteroatom nucleophile reagents in transition metal-catalyzed allylic substitution. For example, molybdenum-catalyzed allylic substitution reactions of heteroatom nucleophiles are unknown and largely limited only to carbon–carbon bond formation procedures (Fig. 1A, left).4 Furthermore, the substrate scope with respect to the allylic electrophile has also remained unchanged and restricted to the ones that provide products containing a tertiary center at the allylic position.5 Regardless of the longstanding interest in the formation of carbon–heteroatom bonds within the synthetic organic community, as well as the advancement of other transition-metal-catalyzed reactions to provide heteroatom bearing quaternary and/or tertiary allylic centers,6 molybdenum-catalyzed allylic substitution reactions that provide products containing such a stereocenter remain prominently absent from the literature and yet to be discovered (Fig. 1A, right).7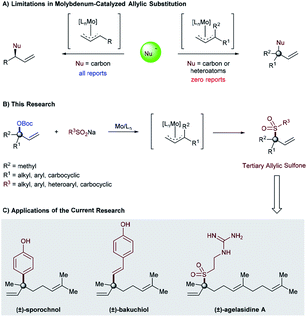 | ||
| Fig. 1 (A) Limitations in molybdenum-catalyzed allylic substitution, (B) our research, and (C) applications of the current research. | ||
Due to the high importance of allylic sulfones as pharmaceuticals8 and synthetic candidates,9 organic chemists have recently been designing catalytic C–S bond cleavage procedures as a new tool for carbon–carbon bond formation through Suzuki cross-coupling10 and/or allylic substitution reactions.11 Despite the considerable development realized in this area, allylic sulfone formation is still a challenging task and confined to the use of transition metal-catalyzed allylic sulfonylation procedures.12,13 However, using these procedures for the synthesis of allylic sulfones containing tetrasubstituted carbon centers is limited and largely unexplored.14 Therefore, at the beginning of our study it was unclear whether a molybdenum-catalyzed allylic substitution could ever be implemented with a heteroatom (sodium sulfinate) nucleophile or even with α,α-disubstituted allylic precursors. If successful, such unexplored areas of allylic substitution chemistry might not only provide an opportunity to realize currently inaccessible chemical space (carbon–heteroatom bond formation) in molybdenum-catalyzed allylic substitution, but also provide a new synthetic approach for rapidly generating quaternary all-carbon centers through Suzuki cross-coupling of the sulfone functionality. As part of our ongoing program in developing molybdenum-catalyzed allylic substitution technology and our continued interest in the catalytic asymmetric synthesis of quaternary stereocenters,14a,15 we were attracted to this unmet challenge and report herein the successful implementation of this idea (Fig. 1B). The salient features of this process are the atom-economic procedures, high regioselectivity, and excellent functional group tolerance for both sulfinate salt and tertiary allylic carbonates, even in the context of late-stage functionalization. Furthermore, the high reactivity of tertiary allylic sulfones as a new class of electrophiles to yield structurally diverse products containing quaternary all-carbon centers through Suzuki cross-coupling is a special characteristic feature of this catalytic system (Fig. 1C).10a
Results and discussion
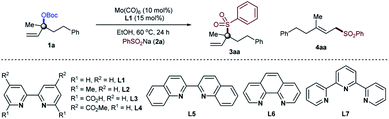
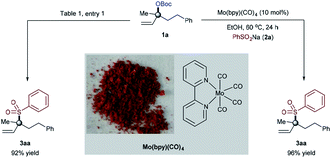
Our optimization began by evaluating the allylic substitution of tertiary allylic carbonate 1a, readily prepared from the corresponding alcohol on a large scale, with sodium benzenesulfinate 2a (Table 1). Interestingly, a disappointing amount of either 3aa or 4aa was detected under reaction conditions previously reported for other molybdenum-catalyzed allylic substitution reactions.4 After several experiments,16 we concluded that a combination of the inexpensive commercially available Mo(CO)6 precursor and 2,2′-bipyridine as a ligand (L1)17 in EtOH at 60 °C afforded 3aa in 92% yield upon isolation with excellent branched to linear selectivity (3aa/4aa = >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Amongst all of the ligands utilized, 2,2′-bipyridine motifs were crucial for achieving the targeted transformation. While excellent reactivity towards 3aa was found with 2,2′-bipyridines and 6,6′-dimethyl-2,2′-bipyridine, better yields were obtained for the first one (entries 1–7). Interestingly, the bench-stable terpyridine L7 failed to provide product 3aa. These results indicate that the coordination geometry of the ligand dictates the reactivity, with 2,2′-bipyridine ligands being particularly suited for the high yield and selectivity of 3aa. Subtle changes in the molybdenum precursor and/or solvent, however, had a negative influence on the reaction, consistently providing lower yields if any (entries 8–14). As anticipated, control experiments revealed that all of the reaction parameters were necessary for the reaction to occur (entry 15).
1). Amongst all of the ligands utilized, 2,2′-bipyridine motifs were crucial for achieving the targeted transformation. While excellent reactivity towards 3aa was found with 2,2′-bipyridines and 6,6′-dimethyl-2,2′-bipyridine, better yields were obtained for the first one (entries 1–7). Interestingly, the bench-stable terpyridine L7 failed to provide product 3aa. These results indicate that the coordination geometry of the ligand dictates the reactivity, with 2,2′-bipyridine ligands being particularly suited for the high yield and selectivity of 3aa. Subtle changes in the molybdenum precursor and/or solvent, however, had a negative influence on the reaction, consistently providing lower yields if any (entries 8–14). As anticipated, control experiments revealed that all of the reaction parameters were necessary for the reaction to occur (entry 15).
| Entry | Deviation in conditions | 3aa/4aab | 3aa (%) |
|---|---|---|---|
| a Reaction conditions: Mo-catalyst (10 mol%), ligand (15 mol%), 1a (0.2 mmol), PhSO2Na 2a (0.3 mmol), solvent (1.0 mL, 0.2 M), 60 °C, 24 hours. b Determined by 1H-NMR of the crude reaction mixture. c Isolated yields. | |||
| 1 | None | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 2 | L2 was used instead of L1 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 3 | L3 was used instead of L1 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35 |
| 4 | L4 was used instead of L1 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52 |
| 5 | L5 was used instead of L1 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 |
| 6 | L6 was used instead of L1 | — | 0 |
| 7 | L7 was used instead of L1 | — | >5 |
| 8 | (C7H8)3Mo(CO)3 was used | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82 |
| 9 | THF was used as solvent | — | >5 |
| 10 | Toluene was used as solvent | — | >5 |
| 11 | DCE was used as solvent | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35 |
| 12 | iPrOH was used as solvent | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 |
| 13 | THF/EtOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent 1) as solvent |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 14 | DCE/EtOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent 1) as solvent |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63 |
| 15 | Without Mo or L1 | — | 0 |
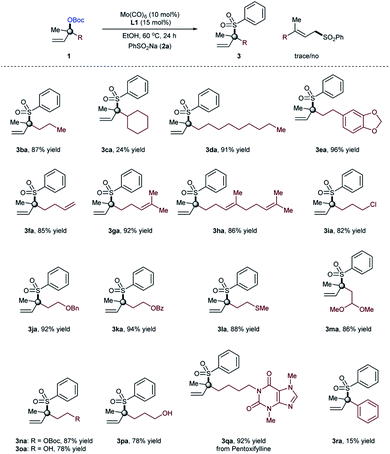
With reliable access to 3aa, we next turned our attention to examine the generality of our newly developed molybdenum-catalyzed regioselective sulfonylation of tertiary allylic electrophiles with sodium sulfinate by using the Mo/L1 catalyst system as shown in Table 2. In all cases analysed for sulfinate salts (2), excellent reactivity and selectivity was observed. Both the electron-withdrawing and electron-donating substituents on the aromatic ring of the sulfinate salts react smoothly with 1a, affording the corresponding α,α-disubstituted allylic products in high yields (3aa–3ap). Sodium sulfinates with bulky naphthyl (3aq), quinoline (3ar), 2,3-dihydrobenzofuran (3as), and heteroaryl (3at, 3au) moieties were also tolerated under the current optimized conditions. Likewise, the targeted tertiary allylic sulfone formation could be extended to sulfinate salts with alkyl substituents. Both primary and secondary alkyl substituted sodium sulfinates worked well to provide α,α-disubstituted allylic sulfones in high yields (3av–3ay). Furthermore, a more functionalized sodium sulfinate 2z, when used as the sulfonylation partner, the branched product 3az was obtained in 72% of isolated yield. The reaction leading to tertiary allylic sulfone 3aa was easily scaled up to gram-scale without significant decrease in yield. Of particular note is that, almost in all cases, the reactions proceeded with excellent branched regioselectivity (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| Entry | 2 | 3 | Yieldc (%) |
|---|---|---|---|
| a Reaction conditions: Mo(CO)6 (10 mol%), L1 (15 mol%), 1a (0.2 mmol), RSO2Na 2 (0.3 mmol), EtOH (1.0 mL, 0.2 M), 60 °C, 24 hours. b Determined by 1H-NMR of the crude reaction mixture. c Isolated yields. | |||
| 1 | 2a (R = Ph) | 3aa | 92 |
| 2 | 2b (R = 4-MeC6H4) | 3ab | 93 |
| 3 | 2c (R = 4-MeOC6H4) | 3ac | 90 |
| 4 | 2d (R = 4-ClC6H4) | 3ad | 87 |
| 5 | 2e (R = 4-FC6H4) | 3ae | 85 |
| 6 | 2f (R = 4-NO2C6H4) | 3af | 75 |
| 7 | 2g (R = 4-CNC6H4) | 3ag | 72 |
| 8 | 2h (R = 2-FC6H4) | 3ah | 88 |
| 9 | 2i (R = 2-ClC6H4) | 3ai | 87 |
| 10 | 2j (R = 2-OCF3C6H4) | 3aj | 72 |
| 11 | 2k (R = 3-BrC6H4) | 3ak | 82 |
| 12 | 2l (R = 3-CNC6H4) | 3al | 78 |
| 13 | 2m (R = 2,4-MeOC6H3) | 3am | 94 |
| 14 | 2n (R = 3,5-CF3C6H3) | 3an | 95 |
| 15 | 2o (R = 2-MeO, 5-BrC6H3) | 3ao | 84 |
| 16 | 2p (R = 3,4-ClC6H3) | 3ap | 87 |
| 17 | 2q (R = 2-naphthyl) | 3aq | 82 |
| 18 | 2r (R = 1-quinoline) | 3ar | 78 |
| 19 | 2s (R = 2,3-dihydrobenzofuran) | 3as | 92 |
| 20 | 2t (R = 3-pyridine) | 3at | 82 |
| 21 | 2u (R = 2-thiophene) | 3au | 86 |
| 22 | 2v (R = Me) | 3av | 72 |
| 23 | 2w (R = Et) | 3aw | 78 |
| 24 | 2x (R = iPr) | 3ax | 82 |
| 25 | 2y (R = cyclopropyl) | 3ay | 78 |
| 26 | 2z (R = CH3OCOCH2CH2) | 3az | 72 |
We then focused on investigating the scope of the α,α-disubstituted allylic carbonates and the results obtained were compiled in Table 3. Tertiary allylic carbonate with simple propyl substituent (1b) reacted efficiently with sodium benzenesulfinate (2a) to deliver the branched allylic sulfone 3ba in high yield (87%). However, allylic carbonate with a cyclohexyl moiety afforded the desired branched product in comparatively low yield (24%, 3ca) due to the steric hindrance problem. However, tertiary allylic carbonate (1d) having a longer alkyl chain provided the desired product even at high yield (91%, 3da). When tertiary allylic carbonates 1e, 1f, 1g and 1h with different groups on the alkyl chain were coupled with sulfinate salt 2a, high yields of the branched allylic products were obtained (85–96%, 3ea, 3fa, 3ga and 3ha). Notably, various common functional groups such as Cl (1i), benzyl (1j), benzoyl (1k), thioether (1l), acetal (1m), and carbonate (1n) on the alkyl chain of the tertiary allylic carbonates were tolerated, and the sulfonylation branched products (3ia–3na) were isolated in high yields (82–94%). In addition, the unprotected hydroxy group on the alkyl chain of the tertiary allylic carbonates 1o and 1p do not interfere with productive tertiary allylic sulfone formation (3oa and 3pa), thus providing opportunities for further derivatization. Notably, the reaction can be easily applied within the context of late-stage functionalization, supported by the formation of branched allylic sulfone 3qa, derived from pentoxifylline. As expected, the allylic sulfonylation of phenyl substituted allylic carbonate occurred exclusively at the less-hindered position. The present optimized conditions were unsatisfactory with such substrates and provided the desired branched product (3ra) with a low branched to linear ratio (b/l = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); indicating some (steric) limitation of the current protocol. Besides methyl-substituted tertiary allylic substrates 1a–1r, other alkyl or aryl substituted substrates provide only starting materials when used under the optimized conditions, indicating some limitation of the present protocol.
5); indicating some (steric) limitation of the current protocol. Besides methyl-substituted tertiary allylic substrates 1a–1r, other alkyl or aryl substituted substrates provide only starting materials when used under the optimized conditions, indicating some limitation of the present protocol.
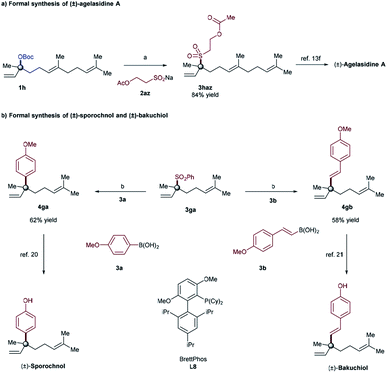
In order to illustrate the synthetic utility of these elusive tertiary allylic sulfones, we focused on the reaction of α,α-disubstituted allylic carbonate (1h), and sodium sulfinate 2az, to achieve the formal synthesis of (±)-agelasidine A.18 The desired tertiary allylic sulfone 3haz was isolated in 84% yield under the standard conditions (Fig. 1A). This compound (3haz) can be readily converted to (±)-agelasidine A by following the literature procedure.13f We further demonstrate that the current methodology can be utilized to prepare other related compounds containing sulfone-bearing quaternary carbon centers.19
Due to their ambiphilic nature, allylic sulfones are synthetically important electrophiles and have recently been utilized in Suzuki cross-coupling10a as well as allylic substitution reactions.11d However, selective cross-coupling of tertiary allylic sulfones remains highly challenging in Suzuki–Miyaura cross-coupling reactions.10 Indeed, we employed our tertiary allylic sulfone product 3ga along with typical boronic acids as a coupling partner in order to achieve the formal synthesis of (±)-sporochnol,20 and (±)-bakuchiol,21 both of which are natural products possessing a quaternary all-carbon center. Our synthesis is illustrated in Fig. 1B. The key step involves a previously reported Suzuki–Miyaura cross-coupling reaction of tertiary allylic sulfone 3ga to afford 4ga, and 4gb efficiently with 62% and 58% of isolated yields respectively. Subsequent deprotection of phenol then could complete the formal synthesis of (±)-sporochnol and (±)-bakuchiol (Fig. 1B).20,21 Starting from 3ga in 2 steps our tertiary allylic sulfones can be used to prepare such natural products and other related compounds bearing all-carbon quaternary centers in a modular way.22
To gain mechanistic insight and the initial understanding on how the reaction works, we decided to study the reactivity of [Mo0Ln] species (Fig. 3). The [Mo(bpy)(CO)4] complex23 was prepared on a large scale by reacting Mo(CO)6 and 2,2′-bipyridine (L1) in THF at 60 °C.16 As shown in Fig. 3, the structure was confirmed and further analyzed.24 Interestingly, the [Mo(bpy)(CO)4] complex was found to be catalytically more efficient when used under the standard conditions, supported by the formation of branched allylic sulfone product 3aa in 96% yield. A small decline in yield of 3aa in the [Mo(CO)6]/L1 catalyst system was observed, thus providing evidence that a [Mo(bpy)(CO)4] complex is likely to be the active precatalyst species in this allylic sulfonylation reaction.25
Conclusions
In conclusion we have developed a method for the allylic sulfonylation of α,α-disubstituted allylic electrophiles, using inexpensive and commercially available catalyst components (Mo(CO)6/2,2′-bipyridine). To the best of our knowledge, the presented methodology is the first example of the use of sodium sulfinates as the heteroatom nucleophile reagent with tertiary allylic electrophiles to employ the group 6 catalyst in allylic substitution of tertiary allylic electrophiles to form C–S bonds. The process is characterized by its atom economic procedure, wide substrate scope, and excellent regioselectivity profile even in the context of late-stage functionalization, thus providing ample opportunities for further derivatization through traditional Suzuki cross-coupling reactions (as presented in Fig. 2b). Investigations of enantioselective reactions, the mechanism and extension to other heteroatom nucleophiles are currently ongoing and will be reported in due course.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the “Fundamental Research Funds for Central Universities” (No. 1191329135), Key Laboratory Construction Program of Xi'an Municipal Bureau of Science and Technology (No. 201805056ZD7CG40) and the starting fund of Xi'an Jiao Tong University (No. 7121182002). We thank the Instrumental Analysis Center of Xi'an Jiao Tong University for HRMS analysis.Notes and references
- (a) J. Tsuji, H. Takahashi and M. Morikawa, Tetrahedron Lett., 1965, 6, 4387 CrossRef; (b) B. M. Trost and T. J. Fullerton, J. Am. Chem. Soc., 1973, 95, 292 CrossRef CAS.
- For selected reviews on the applications of allylic substitutions, see: (a) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed; (b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (c) Z. Lu and S.-M. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed; (d) J. D. Weaver, A. Recio, A. J. Grenning and J. A. Tunge, Chem. Rev., 2011, 111, 1846 CrossRef CAS PubMed; (e) L. Milhau and P. J. Guiry, Top. Organomet. Chem., 2011, 38, 95 CrossRef CAS; (f) B. Sundararaju, M. Achard and C. Bruneau, Chem. Soc. Rev., 2012, 41, 4467 RSC; (g) G. Cheng, H.-F. Tu, C. Zheng, J.-P. Qu, G. Helmchen and S.-L. You, Chem. Rev., 2019, 119, 1855 CrossRef PubMed.
- For selected reviews on heteroatom nucleophiles, see: (a) M. Johannsen and K. A. Jørgensen, Chem. Rev., 1998, 98, 1689 CrossRef CAS PubMed; (b) B. M. Trost, T. Zhang and J. D. Sieber, Chem. Sci., 2010, 1, 427 RSC; (c) L. Huang, M. Arndt, K. Gooßen, H. Heydt and L. J. Gooßen, Chem. Rev., 2015, 115, 2596–2697 CrossRef CAS PubMed; (d) R. L. Grange, E. A. Clizbe and P. A. Evans, Synthesis, 2016, 48, 2911 CrossRef CAS; (e) R. Takeuchi and S. Kezuka, Synthesis, 2006, 20, 3349 CrossRef; (f) J. F. Hartwig and L. M. Stanley, Acc. Chem. Res., 2010, 43, 1461 CrossRef CAS PubMed.
- For selected reviews on Mo-catalysed allylic alkylation reactions, see: (a) O. Belda and C. Moberg, Acc. Chem. Res., 2004, 37, 159 CrossRef CAS PubMed; (b) C. Moberg, Top. Organomet. Chem., 2011, 38, 209 CrossRef CAS; (c) B. M. Trost, Org. Process Res. Dev., 2012, 16, 185 CrossRef CAS PubMed; (d) C. Moberg, Org. React., 2014, 84, 1 CAS. For original examples, see: (e) B. M. Trost and I. Hachiya, J. Am. Chem. Soc., 1998, 120, 1104 CrossRef CAS; (f) A. V. Malkov, L. Gouriou, G. C. Lloyd-Jones, I. Stary, V. Langer, P. Spoor, V. Vinader and P. Kocovsky, Chem.–Eur. J., 2006, 12, 6910 CrossRef CAS PubMed; (g) B. M. Trost and K. Dogra, J. Am. Chem. Soc., 2002, 124, 7256 CrossRef CAS PubMed; (h) S. W. Krska, D. L. Hughes, R. A. Reamer, D. J. Mathre and Y. Sun, J. Am. Chem. Soc., 2002, 124, 12656 CrossRef CAS PubMed; (i) B. M. Trost and Y. Zhang, J. Am. Chem. Soc., 2007, 129, 14548 CrossRef CAS PubMed; (j) B. M. Trost and Y. Zhang, Chem.–Eur. J., 2010, 16, 296 CrossRef CAS PubMed; (k) B. M. Trost and Y. Zhang, Chem.–Eur. J., 2011, 17, 2916 CrossRef CAS PubMed; (l) B. M. Trost, J. R. Miller and C. M. Hoffman, J. Am. Chem. Soc., 2011, 133, 8165 CrossRef CAS PubMed; (m) E. Ozkal and M. A. Pericas, Adv. Synth. Catal., 2014, 356, 711 CrossRef CAS; (n) B. M. Trost, M. Osipov, S. Kruger and Y. Zhang, Chem. Sci., 2015, 6, 349 RSC.
- An attempted example of the Mo-catalyzed allylic alkylation reaction utilizing a tertiary allylic substrate has been mentioned with unclear results (sluggish reactivity), see: P. Kočovský, A. V. Malkov, S. Vyskočil and G. C. Lloyd-Jones, Pure Appl. Chem., 1999, 71, 1425 Search PubMed.
- For selective examples on the synthesis of quaternary, and/or tertiary allylic compounds with heteroatom nucleophiles, see: (a) B. M. Trost, R. C. Bunt, R. C. Lemoine and T. L. Calkins, J. Am. Chem. Soc., 2000, 122, 5968 CrossRef CAS; (b) D. F. Fisher, Z.-Q. Xin and R. Peters, Angew. Chem., Int. Ed., 2007, 46, 7704 CrossRef PubMed; (c) J. S. Arnold and H. M. Nguye, J. Am. Chem. Soc., 2012, 134, 8380 CrossRef CAS PubMed; (d) B. W. H. Turnbull and P. A. Evans, J. Org. Chem., 2018, 83, 11463 CrossRef CAS PubMed; (e) W. Guo, A. Cai, J. Xie and A. W. Kleij, Angew. Chem., Int. Ed., 2017, 56, 11797 CrossRef CAS PubMed; (f) J. Cai, W. Guo, L. Martínez-Rodríguez and A. W. Kleij, J. Am. Chem. Soc., 2016, 138, 14194 CrossRef PubMed; (g) J. Xie, W. Guo, A. Cai, E. C. Escudero-Adán and A. W. Kleij, Org. Lett., 2017, 19, 6388 CrossRef CAS PubMed; (h) S. Mizuno, S. Terasaki, T. Shinozawa and M. Kawatsura, Org. Lett., 2017, 19, 504 CrossRef CAS PubMed; (i) J. Long, L. Shi, X. Li, H. Lv and X. Zhang, Angew. Chem., Int. Ed., 2018, 57, 13248 CrossRef CAS PubMed; (j) J. E. Gómez, A. Cristòfol and A. W. Kleij, Angew. Chem., Int. Ed., 2019, 58, 3903 CrossRef PubMed; (k) S. Ghorai, S. S. Chirke, W.-B. Xu, J.-F. Chen and C. Li, J. Am. Chem. Soc., 2019, 141, 11430 CrossRef CAS PubMed; (l) T. Sandmeier, F. W. Goetzke, S. Krautwald and E. M. Carreira, J. Am. Chem. Soc., 2019, 141, 12212 CrossRef CAS PubMed; (m) M. Lafrance, M. Roggen and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3470 CrossRef CAS PubMed; (n) M. Roggen and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 8652 CrossRef PubMed; (o) S. L. Rössler, D. A. Petrone and E. M. Carreira, Acc. Chem. Res., 2019, 52, 2657 CrossRef PubMed , and references therein.
- The range of nucleophiles that have been used in molybdenum-catalysed allylic alkylations is limited to stabilized carbon nucleophiles and is thus narrower than that in the palladium- and iridium-catalysed reactions. However, to our knowledge there is no report available in the literature on the use of heteroatom nucleophiles that provide access to tertiary and/or secondary allylic products in Mo-catalysed allylic substitution reactions..
- For the application of sulfones in drugs and bioactive compounds, see: (a) H. Liu and X. Jiang, Chem.–Asian J., 2013, 8, 2546 CrossRef CAS PubMed; (b) M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200 CrossRef CAS PubMed; (c) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed; (d) N. Wang, P. Saidhareddy and X. Jiang, Nat. Prod. Rep., 2020, 37, 246 RSC.
- For the application of sulfones in organic synthesis, see: (a) P. L. Fuchs and T. F. Braish, Chem. Rev., 1986, 86, 903 CrossRef CAS; (b) B. M. Trost, M. G. Organ and G. A. O'Doherty, J. Am. Chem. Soc., 1995, 117, 9662 CrossRef CAS; (c) B. M. Trost, Bull. Chem. Soc. Jpn., 1988, 61, 107 CrossRef CAS; (d) T. Zhou, B. Peters, M. F. Maldonado, T. Govender and P. G. Andersson, J. Am. Chem. Soc., 2012, 134, 13592 CrossRef CAS PubMed; (e) B. K. Peters, T. Zhou, J. Rujirawanich, A. Cadu, T. Singh, W. Rabten, S. Kerdphon and P. W. Andersson, J. Am. Chem. Soc., 2014, 136, 16557 CrossRef CAS PubMed.
- (a) Z. T. Arika, Y. Maekawa, M. Nambo and C. M. Crudden, J. Am. Chem. Soc., 2018, 140, 78 CrossRef PubMed; (b) M. Nambo and C. M. Crudden, Angew. Chem., Int. Ed., 2014, 53, 742 CrossRef CAS PubMed; (c) M. Nambo and C. M. Crudden, ACS Catal., 2015, 5, 4734 CrossRef CAS; (d) M. Nambo, Z. T. Ariki, D. Canseco-Gonzalez, D. D. Beattie and C. M. Crudden, Org. Lett., 2016, 18, 2339 CrossRef CAS PubMed; (e) M. Nambo, E. C. Keske, J. P. G. Rygus, J. C.-H. Yim and C. M. Crudden, ACS Catal., 2017, 7, 1108 CrossRef CAS; (f) J. C.-H. Yim, M. Nambo and C. M. Crudden, Org. Lett., 2017, 19, 3715 CrossRef CAS PubMed.
- (a) B. M. Trost, Bull. Chem. Soc. Jpn., 1988, 61, 107 CrossRef CAS; (b) B. M. Trost and C. A. Merlic, J. Org. Chem., 1990, 55, 1127 CrossRef CAS; (c) B. M. Trost, N. R. Schmuff and M. J. Miller, J. Am. Chem. Soc., 1980, 102, 5979 CrossRef CAS; (d) K. Takizawa, T. Sekino, S. Sato, T. Yoshino, M. Kojima and S. Matsunaga, Angew. Chem., Int. Ed., 2019, 58, 9199 CrossRef CAS PubMed.
- For the synthesis of allylic sulfones via transition metal-catalysed allylic substitution, see: (a) K. Hiroi and K. Makino, Chem. Lett., 1986, 15, 617 CrossRef; (b) H. Eichelmann and H.-J. Gais, Tetrahedron: Asymmetry, 1995, 6, 643 CrossRef CAS; (c) B. M. Trost, M. J. Krische, R. Radinov and G. J. Zanoni, J. Am. Chem. Soc., 1996, 118, 6297 CrossRef CAS; (d) B. M. Trost, M. L. Crawley and C. B. Lee, J. Am. Chem. Soc., 2000, 122, 6120 CrossRef CAS; (e) Y. Uozumi and T. Suzuka, Synthesis, 2008, 12, 1960 CrossRef; (f) M. Jegelka and B. Plietker, Org. Lett., 2009, 11, 3462 CrossRef CAS PubMed; (g) J. A. Wolfe and S. R. Hitchcock, Tetrahedron: Asymmetry, 2010, 21, 2690 CrossRef CAS; (h) M. Ueda and J. F. Hartwig, Org. Lett., 2010, 12, 92 CrossRef CAS PubMed; (i) X.-S. Wu, Y. Chen, M.-B. Li, M.-G. Zhou and S.-K. Tian, J. Am. Chem. Soc., 2012, 134, 14694 CrossRef CAS PubMed; (j) T.-T. Wang, F.-X. Wang, F.-L. Yang and S.-K. Tian, Chem. Commun., 2014, 50, 3802 RSC; (k) X.-T. Ma, R.-K. Dai, J. Zhang, Y. Gu and S.-K. Tian, Adv. Synth. Catal., 2014, 356, 2984 CrossRef CAS; (l) A. Najib, K. Hirano and M. Miura, Chem.–Eur. J., 2018, 24, 6525 CrossRef CAS PubMed.
- For selected examples on hydrothiolation of allenes, see: (a) A. B. Pritzius and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 3121 CrossRef CAS PubMed; (b) A. B. Pritzius and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 15818 CrossRef CAS PubMed. For the direct hydrosulfination of allenes and alkynes, see: (c) K. Xu, V. Khakyzadeh, T. Bury and B. Breit, J. Am. Chem. Soc., 2014, 136, 16124 CrossRef CAS PubMed; (d) V. Khakyzadeh, Y.-H. Wang and B. Breit, Chem. Commun., 2017, 53, 4966 RSC. For selected examples on hydrothiolation of olefins and dienes, see: (e) E. Mosaferi, D. Ripsman and D. W. Stephan, Chem. Commun., 2016, 52, 8291 RSC; (f) X.-H. Yang, R. T. Davison, S.-Z. Nie, F. A. Cruz, T. M. McGinnis and V. M. Dong, J. Am. Chem. Soc., 2019, 141, 3006 CrossRef CAS PubMed.
- The synthesis of α,α-disubstituted allylic sulfones through transition metal catalyzed procedures is limited to the recent two examples reported by us and others, see: (a) A. Khan, M. Zhang and S. Khan, Angew. Chem., Int. Ed., 2020, 59, 1340 CrossRef CAS PubMed; (b) A. Cai and A. W. Kleij, Angew. Chem., Int. Ed., 2019, 58, 14944 CrossRef CAS PubMed.
- (a) A. Khan, S. Khan, I. Khan, C. Zhao, Y. Mao, Y. Chen and Y. J. Zhang, J. Am. Chem. Soc., 2017, 139, 10733 CrossRef CAS PubMed; (b) A. Khan, R. Zheng, Y. Kan, J. Ye, J. Xing and Y. J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 6439 CrossRef CAS PubMed; (c) A. Khan, L. Yang, J. Xu, L. Y. Jin and Y. J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 11257 CrossRef CAS PubMed; (d) A. Khan, J. Xing, J. Zhao, Y. Kan, W. Zhang and Y. J. Zhang, Chem.–Eur. J., 2015, 21, 120 CrossRef CAS PubMed.
- For details, see ESI.†.
- For reviews on the use of bipyridine type ligands, see; (a) C. Kaes, A. Katz and M. W. Hosseini, Chem. Rev., 2000, 100, 3553 CrossRef CAS PubMed; (b) G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129 CrossRef CAS PubMed.
- H. Nakamura, H. Wu, J. Kobayashi, Y. Ohizumi, Y. Hirata, T. Higashijima and T. Miyazawa, Tetrahedron Lett., 1983, 24, 4105 CrossRef CAS.
- E. P. Stout, L. C. Yu and T. F. Molinski, Eur. J. Org. Chem., 2012, 2012, 5131 CrossRef CAS PubMed.
- (a) S. Shan and C. Ha, Synth. Commun., 2004, 34, 4005 CrossRef CAS; (b) Y. Li, J. Han, H. Luo, Q. An, X.-P. Cao and B. Li, Org. Lett., 2019, 21, 6050 CrossRef CAS PubMed; (c) M. Kacprzynski and A. H. Hoveyda, J. Am. Chem. Soc., 2004, 126, 10676 CrossRef CAS PubMed; (d) R. P. Sonawane, V. Jheengut, C. Rabalakos, R. Larouche-Gauthier, H. K. Scott and V. K. Aggarwal, Angew. Chem., Int. Ed., 2011, 50, 3760 CrossRef CAS PubMed.
- (a) R. Majeed, M. V. Reddy, P. K. Chinthakindi, P. L. Sangwan, A. Hamid, G. Chashoo, A. K. Saxena and S. Koul, Eur. J. Med. Chem., 2012, 49, 55 CrossRef CAS PubMed; (b) Y. Xiong and G. Zhang, Org. Lett., 2016, 18, 5094 CrossRef CAS PubMed; (c) F. Gao, K. P. McGrath, Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2010, 132, 14315 CrossRef CAS PubMed; (d) S. Chakrabarty and J. M. Takacs, J. Am. Chem. Soc., 2017, 139, 6066 CrossRef CAS PubMed.
- N. F. Fine Nathel, T. K. Shah, S. M. Bronner and N. K. Garg, Chem. Sci., 2014, 5, 2184 RSC.
- (a) K. R. Birdwhistell, B. E. Schulz and P. M. Dizon, Inorg. Chem. Commun., 2012, 26, 69 CrossRef CAS; (b) G. Neri, P. M. Donaldson and A. J. Cowan, J. Am. Chem. Soc., 2017, 139, 13791 CrossRef CAS PubMed.
- Despite considerable efforts, we were unable to prepare a single crystal of Mo(bpy)(CO)4. At present, we have confirmed the structure from NMR-analysis. All the spectroscopic data for this compound match with those reported in the literature. See ref. 16 and 23 for more details.
- For mechanistic hypothesis, see: (a) B. M. Trost and M. Lautens, J. Am. Chem. Soc., 1982, 104, 5543 CrossRef CAS; (b) B. M. Trost and M. H. Hung, J. Am. Chem. Soc., 1983, 105, 7757 CrossRef CAS; (c) D. E. Ryan, D. J. Cardin and F. Hartl, Coord. Chem. Rev., 2017, 335, 103 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: For detailed experimental procedures, characterization data of all of the new compounds, and copies of 1H, 13C NMR spectra of the substrates and products. See DOI: 10.1039/d0sc01763a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |