Open Access Article
Open Access ArticlePhoto-induced carbocation-enhanced charge transport in single-molecule junctions†
Zhongwu
Bei
b,
Yuan
Huang
b,
Yangwei
Chen
b,
Yiping
Cao
 *b and
Jin
Li
*b and
Jin
Li
 *a
*a
aResearch Center for Analytical Sciences, College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: rengong0225@163.com
bKey Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education, Jianghan University, Wuhan 430056, China
First published on 22nd May 2020
Abstract
We report the first example of photo-induced carbocation-enhanced charge transport in triphenylmethane junctions using the scanning tunneling microscopy break junction (STM-BJ) technique. The electrical conductance of the carbocation state is enhanced by up to 1.5 orders of magnitude compared to the initial state, with stability lasting for at least 7 days. Moreover, we can achieve light-induced reversible conductance switching with a high ON–OFF ratio in carbocation-based single-molecule junctions. Theoretical calculations reveal that the conductance increase is due to a significant decrease of the HOMO–LUMO gap and also the enhanced transmission close to the Fermi levels when the carbocation forms. Our findings encourage continued research toward developing optoelectronics and carbocation-based devices at the single-molecule level.
Introduction
Since its inception, molecular electronics has aimed for functional electronic devices using individual molecules.1 Understanding and investigations of charge transport through single molecules provide critical information for the design of molecular-scale devices.2–5 Various molecular species are being employed in single-molecule devices for regulating charge transport properties in single-molecule junctions, for instance oligoynes,6 oligo(phenylene ethynylene),7 organic radicals,8 DNA,9 and peptides.10 Although carbocations have been widely found in many chemical reactions,11–13 to date, the applications of carbocation-based molecules as building blocks to fabricate stable and highly conductive molecular devices remain highly challenging due to the intrinsic instability of a majority of carbocations.On the other hand, an external stimulus for tuning charge transport through molecular junctions also plays a key role in the fabrication of single-molecule devices. Previously several external stimuli such as light,14 pH,15 electric fields,16 mechanical forces,17 solvents,18 and electrochemical gates19 were used to tailor the electronic properties of single-molecule junctions. Among these stimuli, light has an advantage in terms of its remote manner, non-invasiveness, and high spatiotemporal resolution.20 Therefore the utilization of light to tune charge transport may provide a unique strategy for creating new conceptual molecular devices.
Triphenylmethane leuco derivatives are well-known photochromic molecules, which dissociate into ion pairs under ultraviolet irradiation, producing stable carbocations in the form of triarylmethane dyes and hydroxide ions.21–23 The stable carbocation dyes have re-emerged as a highly efficient Lewis acid catalysts for a variety of organic transformations. Investigations of this reaction have revealed that the dissociation processes are very fast and proceed with high quantum yields.22 Such prominent changes in pH have received wide interests as light-induced pH-jump reactions for many applications,22,23 and also offer potential opportunities to study carbocation-based charge transport properties at the single-molecule level.
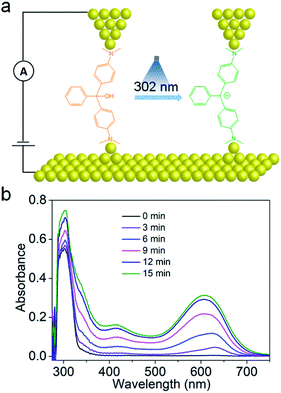
In the present work, we selected malachite green leucohydroxide (MGOH) molecules as carbocation emitters to explore laser-induced charge transport in single-molecule junctions. Using the scanning tunneling microscopy break junction (STM-BJ) technique,24 the single molecule conductance of MGOH and the corresponding junction elongation can be evaluated (Fig. 1a). The dimethylamino moieties at both ends of MGOH serving as the anchoring groups are used to build efficient transport junctions. In the presence of 302 nm UV light, MGOH produces, with high efficiency, malachite green carbocations (MG+) and hydroxide ions, thus inducing a minor structural change where the orbital hybridization of the central carbon atom of initial states changes from sp3 to sp2. Such a photo-triggered structural transformation shifts the HOMO–LUMO gap and we demonstrate that the carbocations significantly enhance the conductance and the conductance switching could be highly reversible. The large enhancement in single-molecule conductance suggests that the transport pathway of charges in the carbocation state is markedly different to that of initial states, and it is further supported by the DFT calculations that Breit–Wigner resonance occurs in the carbocation state, leading to a large ON–OFF ratio in the junctions.
Results and discussion
To confirm the formation of the carbocations, we performed UV-Vis absorption spectroscopy of MGOH before and after UV illumination as shown in Fig. 1b. Before illumination, MGOH exhibits a single peak at 299 nm. After illumination at 25 °C, two prominent peak bands at 416 nm and 610 nm appeared and their intensities progressively increased with time, which correspond to the characteristic of the triphenylmethane carbocation.16,18In situ photo-radiation of MGOH results in the orbital hybridization of the central carbon atom changing from sp3 to sp2. The formation of the carbocation states is also confirmed by 1H-NMR spectra (Fig. S1 in the ESI†) and ESI-MS results (Fig. S2 and S3 in the ESI†).To investigate the charge transport properties through the single-molecule carbocation-based junctions, we utilized a home-built STM-BJ technique for trapping single molecules in the mixed solvent of THF and TMB, (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
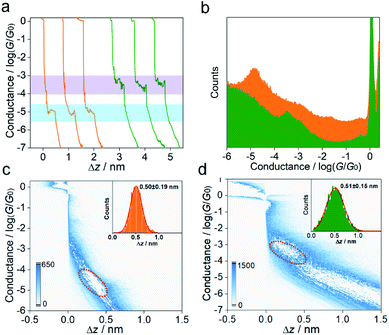
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) (see the ESI† for more details of single-molecule conductance measurements and Fig. S4† for the STM-BJ setup and Fig. S5† for blank experiment results of the pure solvent). Fig. 2a presents typical conductance–distance traces obtained from MGOH and carbocations. Before illumination, a low conductance at ∼10−4.97G0 (0.8 nS) with a plateau length of ∼0.5 nm is observed, indicating the formation of single-molecule junctions comprising MGOH, where G0 (equals 2e2/h, 77.5 μS) corresponds to the conductance quantum of a single gold–gold atomic junction. After illumination with 302 nm light for 15 min, a higher conductance of ∼10−3.43G0 (28.8 nS) with a similar step length is observed.
4, v/v) (see the ESI† for more details of single-molecule conductance measurements and Fig. S4† for the STM-BJ setup and Fig. S5† for blank experiment results of the pure solvent). Fig. 2a presents typical conductance–distance traces obtained from MGOH and carbocations. Before illumination, a low conductance at ∼10−4.97G0 (0.8 nS) with a plateau length of ∼0.5 nm is observed, indicating the formation of single-molecule junctions comprising MGOH, where G0 (equals 2e2/h, 77.5 μS) corresponds to the conductance quantum of a single gold–gold atomic junction. After illumination with 302 nm light for 15 min, a higher conductance of ∼10−3.43G0 (28.8 nS) with a similar step length is observed.
To ensure these features are statistically reproducible, we repeated the measurements thousands of times and compiled the traces into one-dimensional (1D) and two-dimensional (2D) conductance histograms. From the 1D conductance histograms in Fig. 2b, we observed that the molecular conductance peak for MGOH appeared at ∼10−4.91G0 (0.9 nS), while the molecular conductance for MG+ at ∼10−3.40G0 (30.9 nS) is 34 times higher than that of MGOH. The corresponding 2D histograms for MGOH and carbocations are displayed in Fig. 2c and d. Both molecules exhibit distinct molecular plateaus and intensity clouds. The junction elongations for both molecular states show the approximate length of around 0.5 nm (insets of Fig. 2c and d), suggesting the structural change of molecular junctions while the binding configuration of the molecular junctions remained similar. We also calculated the formation probability25 for both molecular junctions, and the results show that the junction formation probabilities are between 40% and 50% (Fig. S6 in the ESI†), indicating that the anchoring ability of both molecule species to Au electrodes is similar.
Control experiments were carried out by measuring the conductance of 4,4′-bis(dimethylamino)triphenylmethane leucohydroxide (LMG). It is found that photo-irradiation of a solution of LMG does not induce any detectable change of conductance within the uncertainty of the measurements (Fig. S7 and S8 in the ESI†). These results present unambiguous evidence that the prominent increase of the conductance in triphenylmethane junctions after illumination is ascribable to the photo-induced formation of carbocations. We noted that LMG has a higher conductance compared to MGOH, and the rigorous and fully theoretical explorations on the high-conductance for LMG need further study.
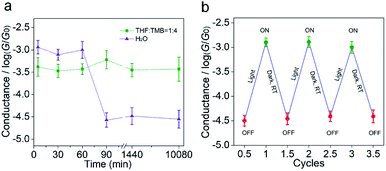
To study the stability of the carbocations, we measured the time-dependent conductance evolution of carbocations in a low-polar solvent (THF/TMB, v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 0.1 mM) that we used and found it was stable for at least 7 days (Fig. 3a). The ESI-MS results and UV-Vis spectrum further indicate that the material is not completely photo-degraded after 7 days (Fig. S9–S12 in the ESI†). The high stability of MG+ is probably due to the fact that sulfonated triphenylmethyl carbocations and hydroxide ions form loose ion pairs in a low-polar solvent.26–28 Previous studies reported that triphenylmethyl carbocations aren't stable and can only exist in water (high-polar) for dozens of minutes.22,23 To further probe the effect of solvent polarity on carbocation stability, we dissolved MGOH molecules in water (0.1 mM), illuminated it with UV light and measured its single-molecule conductance. It is found that the high conductance states lasted for only 60 min (Fig. 3a) in aqueous solution, which is consistent with previous reports, suggesting the stability of carbocations is affected by solvent polarity. By tuning the solvent polarity, the switching could be designed to be irreversible or reversible. In aqueous solutions, the in situ light-induced reactions involving carbocations are reversible and at least three cycles of conductance switching can be achieved as shown in Fig. 3b (Fig. S13 in the ESI†). Carbocation-based junctions in low-polar environments with high stability can serve as conducting interconnects for durable electrical circuits, while carbocation-based junctions with low stability in high-polar environments can act as a light-reversible single-molecule switch. These results suggest carbocations are one of the potential multifunctional building blocks for molecular electronics. Compared with the molecular devices constructed by common molecular systems such as spiropyran29 and azobenzene,30 this work based on carbocation molecular species of interest broadens the scope of building blocks in molecular electronics.
4, 0.1 mM) that we used and found it was stable for at least 7 days (Fig. 3a). The ESI-MS results and UV-Vis spectrum further indicate that the material is not completely photo-degraded after 7 days (Fig. S9–S12 in the ESI†). The high stability of MG+ is probably due to the fact that sulfonated triphenylmethyl carbocations and hydroxide ions form loose ion pairs in a low-polar solvent.26–28 Previous studies reported that triphenylmethyl carbocations aren't stable and can only exist in water (high-polar) for dozens of minutes.22,23 To further probe the effect of solvent polarity on carbocation stability, we dissolved MGOH molecules in water (0.1 mM), illuminated it with UV light and measured its single-molecule conductance. It is found that the high conductance states lasted for only 60 min (Fig. 3a) in aqueous solution, which is consistent with previous reports, suggesting the stability of carbocations is affected by solvent polarity. By tuning the solvent polarity, the switching could be designed to be irreversible or reversible. In aqueous solutions, the in situ light-induced reactions involving carbocations are reversible and at least three cycles of conductance switching can be achieved as shown in Fig. 3b (Fig. S13 in the ESI†). Carbocation-based junctions in low-polar environments with high stability can serve as conducting interconnects for durable electrical circuits, while carbocation-based junctions with low stability in high-polar environments can act as a light-reversible single-molecule switch. These results suggest carbocations are one of the potential multifunctional building blocks for molecular electronics. Compared with the molecular devices constructed by common molecular systems such as spiropyran29 and azobenzene,30 this work based on carbocation molecular species of interest broadens the scope of building blocks in molecular electronics.
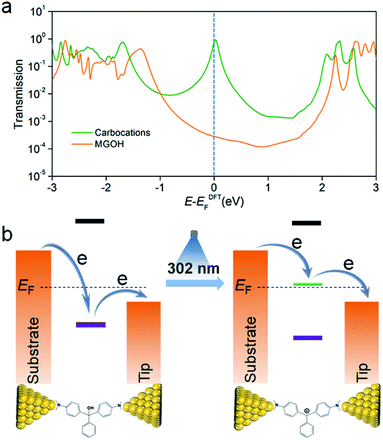
To understand the charge transport properties of initial and carbocation states, we turned to density functional theory (DFT) calculations. We first calculated the Au–N binding energies in both molecular junctions and the results show that the Au–N binding energies for MGOH and the carbocations are respectively −0.89 eV and −0.41 eV (see Fig. S14 and S15 in the ESI†), which imply that dimethylamino moieties in both molecular species can bind to gold electrodes for stable molecular junctions. Next, we calculated their transmission spectra using the non-equilibrium Greens function (NEGF) formalism with DFT.31–33Fig. 4a shows the transmission spectra for MGOH and carbocations, which represent the probability that an electron with given energy will transmit through the molecule between the electrodes (see the conformations of both molecules in Fig. S16 in the ESI†). DFT calculations indicate that the HOMO–LUMO gap changes from 4.65 eV for MGOH to 2.59 eV for MG+ when the carbocation forms (see frontier molecular orbitals and their energy levels in Fig. S17 and Table S1,† and carbocation-based molecular conformations with different counter-ions and different positions and corresponding transmission spectra in Fig. S18 and S19 in the ESI†), which is also confirmed by the red-shift of the UV-Vis spectra in Fig. 1b. Moreover, it is also found that carbocations create a new resonance in the energy level close to Fermi levels (EF), which is responsible for the boosted conductance phenomena through carbocation-based transport junctions. We also note that this resonance in the transmission spectra has a line-shape of the Breit–Wigner distribution.34–36 The transmission for carbocation states at the Fermi level is more than one order of magnitude higher than that for MGOH, which is in good agreement with the experimental observation. Fig. 4b shows a schematic energy diagram of the position of molecular orbitals of carbocations respective to EF of the two electrodes before and after light illumination. Before illumination, the energy-level between molecular orbitals of MGOH and EF is not aligned, suggesting a large energy barrier in charge transport through the HOMO; while a resonance peak is highly aligned to EF in carbocation-based junctions after illumination, which reduces the energy barrier in charge transport and enhances the conductance through LUMO energy lifting. Together, the overall experimental and theoretical studies demonstrate that the in situ light-induced approach produces carbocations, which greatly enhance charge transport in triphenylmethane single-molecule junctions.
Conclusions
In summary, we have demonstrated that carbocations induced by light greatly enhance charge transport in triphenylmethane junctions using the STM-BJ technique. The electrical conductance of the carbocation state is enhanced by up to 1.5 orders of magnitude compared to the initial state and the conductance switching could be reversible. The stable and conductive triphenylmethane carbocations may serve as promising building blocks for future molecular electrical devices. DFT calculations reveal that the conductance increase is due to a significant decrease of the HOMO–LUMO gap and also the enhanced transmission close to the Fermi levels when the carbocation forms. The large conductance enhancement by a light-induced strategy in carbocation-based molecular junctions may be useful for single-molecule optoelectronics and carbocation-based molecular devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Guangdong Basic and Applied Basic Research Foundation (2019A1515011182), and the Natural Science Foundation of the Hubei Province (2019CFB582). We also wish to thank Prof. Wenjing Hong from Xiamen University for helping in single-molecule conductance measurements.Notes and references
- A. Aviram and M. A. Ratner, Chem. Phys. Lett., 1974, 29, 277 CrossRef CAS.
- L. Sun, Y. A. Diaz-Fernandez, T. A. Gschneidtner, F. Westerlund, S. Lara-Avila and K. Moth-Poulsen, Chem. Soc. Rev., 2014, 43, 7378 RSC.
- Y. Zhang, L. Chen, Z. Zhang, J. Cao, C. Tang, J. Liu, L. Duan, Y. Huo, X. Shao, W. Hong and H. Zhang, J. Am. Chem. Soc., 2018, 140, 6531 CrossRef CAS PubMed.
- J. Zheng, J. Liu, Y. Zhuo, R. Li, X. Jin, Y. Yang, Z.-B. Chen, J. Shi, Z. Xiao, W. Hong and Z.-Q. Tian, Chem. Sci., 2018, 9, 5033 RSC.
- B. Xu and N. J. Tao, Science, 2003, 301, 1221 CrossRef CAS PubMed.
- A. Nitzan and M. A. Ratner, Science, 2003, 300, 1384 CrossRef CAS PubMed.
- M. T. González, A. Díaz, E. Leary, R. García, M. Á. Herranz, G. Rubio-Bollinger, N. Martín and N. Agraït, J. Am. Chem. Soc., 2013, 135, 5420 CrossRef PubMed.
- J. Liu, X. Zhao, Q. Al-Galiby, X. Huang, J. Zheng, R. Li, C. Huang, Y. Yang, J. Shi, D. Z. Manrique, C. J. Lambert, M. R. Bryce and W. Hong, Angew. Chem., Int. Ed., 2017, 56, 13061 CrossRef CAS PubMed.
- J. C. Genereux and J. K. Barton, Chem. Rev., 2010, 110, 1642 CrossRef CAS PubMed.
- C. Guo, X. Yua, S. Refaely-Abramsoa, L. Sepunaru, T. Bendikov, I. Pecht, L. Kronik, A. Vilan, M. Sheves and D. Cahen, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 10785 CrossRef CAS PubMed.
- Y. A. Rulev, Z. T. Gugkaeva, A. V. Lokutova, V. I. Maleev, A. S. Peregudov, X. Wu, M. North and Y. N. Belokon, ChemSusChem, 2017, 10, 1152 CrossRef CAS PubMed.
- S. S. Bos and F. E. Treloar, Aust. J. Chem., 1978, 31, 2445 CrossRef CAS.
- R. E. Williams, Chem. Rev., 1992, 92, 177 CrossRef CAS.
- C. Jia, A. Migliore, N. Xin, S. Huang, J. Wang, Q. Yang, S. Wang, H. Chen, D. Wang, B. Feng, Z. Liu, G. Zhang, D.-H. Qu, H. Tian, M. A. Ratner, H. Q. Xu, A. Nitzan and X. Guo, Science, 2016, 352, 1443 CrossRef CAS PubMed.
- Z. Li, M. Smeu, S. Afsari, Y. Xing, M. A. Ratner and E. Borguet, Angew. Chem., Int. Ed., 2014, 53, 1098 CrossRef CAS PubMed.
- F. Schwarz, G. Kastlunger, F. Lissel, C. Egler-Lucas, S. N. Semenov, K. Venkatesan, H. Berke, R. Stadler and E. Lortscher, Nat. Nanotechnol., 2016, 11, 170 CrossRef CAS PubMed.
- I. Franco, C. B. George, G. C. Solomon, G. C. Schatz and M. A. Ratner, J. Am. Chem. Soc., 2011, 133, 2242 CrossRef CAS PubMed.
- D. C. Milan, O. A. Al-Owaedi, M.-C. Oerthel, S. Marqués-González, R. J. Brooke, M. R. Bryce, P. Cea, J. Ferrer, S. J. Higgins, C. J. Lambert, P. J. Low, D. Z. Manrique, S. Martin, R. J. Nichols, W. Schwarzacher and V. M. García-Suárez, J. Phys. Chem. C, 2015, 120, 15666 CrossRef.
- J. Bai, A. Daaoub, S. Sangtarash, X. Li, Y. Tang, Q. Zou, H. Sadeghi, S. Liu, X. Huang, Z. Tan, J. Liu, Y. Yang, J. Shi, G. Meszaros, W. Chen, C. Lambert and W. Hong, Nat. Mater., 2019, 18, 364 CrossRef CAS PubMed.
- S. Cai, W. Deng, F. Huang, L. Chen, C. Tang, W. He, S. Long, R. Li, Z. Tan, J. Liu, J. Shi, Z. Liu, Z. Xiao, D. Zhang and W. Hong, Angew. Chem., Int. Ed., 2019, 58, 3829 CrossRef CAS PubMed.
- C. P. Carvalho, V. D. Uzunova, J. P. Da Silva, W. M. Nau and U. Pischel, Chem. Commun., 2011, 47, 8793 RSC.
- M. Irie, J. Am. Chem. Soc., 1983, 105, 2078 CrossRef CAS.
- H. Liu, Y. Xu, F. Li, Y. Yang, W. Wang, Y. Song and D. Liu, Angew. Chem., Int. Ed., 2007, 46, 2515 CrossRef CAS PubMed.
- M. T. Gonzalez, A. Diaz, E. Leary, R. Garcia, M. A. Herranz, G. Rubio-Bollinger, N. Martin and N. Agrait, J. Am. Chem. Soc., 2013, 135, 5420 CrossRef CAS PubMed.
- C. Zhan, G. Wang, X. Zhang, Z. Li, J. Wei, Y. Si, Y. Yang, W. Hong and Z. Tian, Angew. Chem., Int. Ed., 2019, 131, 14676 CrossRef.
- K. Matyjaszewski, C. Lin, A. Bon and J. S. Xiang, Macromol. Symp., 1994, 85, 65 CrossRef CAS.
- J. P. Richard and W. P. Jencks, J. Am. Chem. Soc., 1982, 104, 4689 CrossRef CAS.
- X. Creary, E. D. Willis and M. Gagnon, J. Am. Chem. Soc., 2005, 127, 18114 CrossRef CAS PubMed.
- N. Darwish, A. C. Aragonés, T. Darwish, S. Ciampi and I. Diez-Perez, Nano Lett., 2014, 14, 7064 CrossRef CAS PubMed.
- J. M. Mativetsky, G. Pace, M. Elbing, M. A. Rampi, M. Mayor and P. Samori, J. Am. Chem. Soc., 2008, 130, 9192 CrossRef CAS PubMed.
- N. R. Claughton, M. Leadbeater and C. J. Lambert, J. Phys.: Condens. Matter, 1995, 7, 8757 CrossRef CAS.
- J. Taylor, H. Guo and J. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245407 CrossRef.
- K. Stokbro, J. Taylor, M. Brandbyge and H. Guo, Ab initio based Non-equilibrium Green's Function Formalism for Calculating Electron Transport in Molecular Devices, Springer, 2005 Search PubMed.
- I. A. Shelykh and N. G. Galkin, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 205328 CrossRef.
- C. J. Lambert, Chem. Soc. Rev., 2015, 44, 875 RSC.
- K. Wang, A. Vezzoli, I. M. Grace, M. McLaughlin, R. J. Nichols, B. Xu, C. J. Lambert and S. J. Higgins, Chem. Sci., 2019, 10, 2396 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc00505c |
| This journal is © The Royal Society of Chemistry 2020 |