Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantio- and diastereoselective diarylmethylation of 1,3-dicarbonyl compounds†
Xin
Li
 ,
Songtao
He
and
Qiuling
Song
,
Songtao
He
and
Qiuling
Song
 *
*
Institute of Next Generation Matter Transformation, College of Materials Science & Engineering, Huaqiao University, 668 Jimei Blvd, Xiamen, Fujian, China. E-mail: qsong@hqu.edu.cn
First published on 25th May 2020
Abstract
A Cu-catalyzed enantio- and diastereoselective diarylmethylation of 1,3-dicarbonyl compounds is described. It is a successful example of constructing all-carbon quaternary stereocenters from 3 °C–H nucleophiles, a challenging topic in synthetic chemistry. In the present work, two contiguous stereocenters are constructed with high levels of stereoselectivity and atom economy. The broad scope of 1,3-dicarbonyl nucleophiles and the tolerance of a wide range of functional groups make this protocol of great importance in the synthesis of chiral diarylmethane compounds.
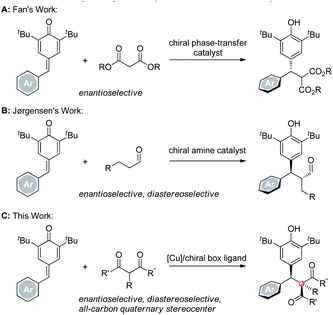
Chiral diarylmethanes are ubiquitous in numerous natural products, medicinal molecules and bioactive compounds.1 As a consequence, many elegant strategies have been developed to construct those structures. Among them, the asymmetric addition of an appropriate nucleophile to an aryl substituted quinone methide is considered as a direct and efficient access to chiral diarylmethanes.2 During the past decade, with the development of many new kinds of nucleophiles and new modes of asymmetric catalytic systems, a variety of C–C3 and C–X4 bonds have been forged to furnish the target chiral diarylmethanes. Among those strategies developed, employing C–H nucleophiles is regarded as an ideal method because 100% atom economy can be achieved in that transformation. For instance, in 2013, Fan reported a beautiful work, in which malonic esters incorporated with para-quinone methides (p-QMs) enantioselectively under a chiral phase-transfer catalytic system (Scheme 1, route A).3a Soon after, Jørgensen demonstrated a new organocatalytic concept to construct two contiguous stereocenters enantio- and diastereoselectively using their iconic chiral secondary amine catalysts (Scheme 1, route B).3b Although great achievements have been made, the next progress in this field, i.e. employing 3° C–H nucleophiles to construct a more challenging all-carbon quaternary stereocenter5 was proven difficult, which may be attributed to the increased steric hindrance and the decreased acidity of the 3° C–H nucleophiles. To date, successful nucleophiles were restricted to oxindoles.6 Therefore, an efficient protocol which highly stereoselectively affords the two stereocenters with a broad 3° C–H nucleophiles scope is demanded. In this communication, we address this problem by employing a series of 1,3-dicarbonyl compounds under a copper-chiral bisoxazoline catalytic system (Scheme 1, route C).
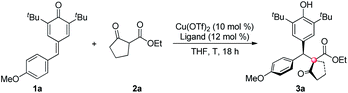
We commenced our investigation by using p-QM 1a and 1,3-dicarbonyl compound 2a as model substrates. After preliminary screening, a combination of 10 mol% of Cu(OTf)2 and 12 mol% of a 1-amino-2-indanol derived bisoxazoline ligand L1 catalyzed the reaction efficiently, affording the desired product with 98% isolated yield, 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
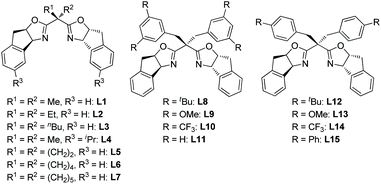
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 78% ee at 0 °C (Table 1, entry 1).7 Based on that result, a structural modification of the ligands family was carried out to improve the stereoselectivity of the reaction (entries 2–16). Gratifyingly, among a small library of 15 synthesized ligands, a 3,5-di-tert-butyl-benzyl substituted ligand L8 gave an excellent outcome (98% isolated yield, 11.5
1 dr and 78% ee at 0 °C (Table 1, entry 1).7 Based on that result, a structural modification of the ligands family was carried out to improve the stereoselectivity of the reaction (entries 2–16). Gratifyingly, among a small library of 15 synthesized ligands, a 3,5-di-tert-butyl-benzyl substituted ligand L8 gave an excellent outcome (98% isolated yield, 11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 94% ee, entry 9). Meanwhile, those data showed that steric factor plays a crucial role in the performance of the ligands. For example, increasing the steric hindrance on the benzene ring of indanol resulted in a dramatic decrease on yield, and slightly decreases on dr and ee (entry 2 vs. entry 5). Besides, ligands bearing 4-substituted benzyl groups all gave much lower yields and poorer diastereoselectivities than their 3,5-disubstituted counterparts (entries 9–11 vs. entries 13–15). Further optimization showed that a higher stereoselectivity can be achieved when the reaction proceeded at −15 °C (entry 17).8
1 dr and 94% ee, entry 9). Meanwhile, those data showed that steric factor plays a crucial role in the performance of the ligands. For example, increasing the steric hindrance on the benzene ring of indanol resulted in a dramatic decrease on yield, and slightly decreases on dr and ee (entry 2 vs. entry 5). Besides, ligands bearing 4-substituted benzyl groups all gave much lower yields and poorer diastereoselectivities than their 3,5-disubstituted counterparts (entries 9–11 vs. entries 13–15). Further optimization showed that a higher stereoselectivity can be achieved when the reaction proceeded at −15 °C (entry 17).8
| Entry | Ligand | T [°C] | Yieldb [%] | drc | eed [%] |
|---|---|---|---|---|---|
| a Reaction conditions: p-quinone methide (0.05 mmol), ethyl 2-oxocyclopentanecarboxylate (0.075 mmol), Cu(OTf)2 (10 mol%), ligand (12 mol%), THF (1.0 mL), 18 h. b Yield of the isolated product. c Determined by 1H-NMR of the crude reaction mixture. d Determined by HPLC analysis and referred to the major diastereoisomer. | |||||
| 1 | L1 | 0 | 98 | 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 |
| 2 | L1 | −10 | 98 | 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 |
| 3 | L2 | −10 | 92 | 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 |
| 4 | L3 | −10 | 90 | 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79 |
| 5 | L4 | −10 | 37 | 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 |
| 6 | L5 | −10 | 24 | 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 |
| 7 | L6 | −10 | 96 | 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 |
| 8 | L7 | −10 | 95 | 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 |
| 9 | L8 | −10 | 98 | 11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 10 | L9 | −10 | 98 | 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 |
| 11 | L10 | −10 | 69 | 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88 |
| 12 | L11 | −10 | 97 | 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 13 | L12 | −10 | 48 | 3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 |
| 14 | L13 | −10 | 40 | 4.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74 |
| 15 | L14 | −10 | 56 | 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 |
| 16 | L15 | −10 | 25 | 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 17 | L8 | −15 | 98 | 14.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
|
|||||
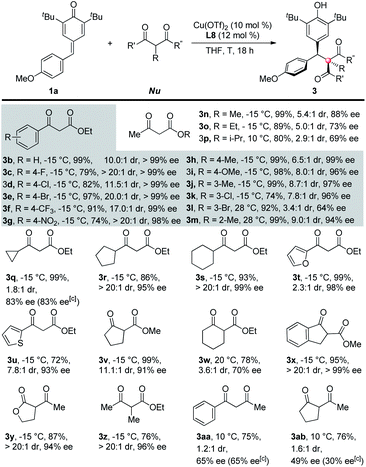
After the optimization works, a series of 1,3-dicarbonyl compounds were submitted to the standard reaction conditions to test the generalizability of the reaction toward C–H nucleophiles (Table 2). For 2 °C–H nucleophiles, ethyl 3-oxo-3-phenylpropanoate derivatives all gave good results with respect to yields, dr and ee's (3b–m). The electronic factor of the benzene rings has little influence on the reactivity and almost no influence on the stereoselectivity. For example, substrates bearing electron-withdrawing substituents on the benzene rings reacted more slowly under the same condition, thus resulting in lower conversions and yields (3c–g, 3k). Nevertheless, both electron-donating and electron-withdrawing substituents gave excellent stereoselectivities (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, up to >99% ee). In contrast, steric factor plays an important role in the reactivity and stereoselectivity of the substrates. For instance, m-bromo substituted substrate (3l) required a higher reaction temperature (ambient temperature)9 to afford the product but with a poorer dr and ee than those bearing smaller substituents such as methyl (3j) or chlorine atom (3k). O-Methyl (3m) also required a higher reaction temperature than m- and p-methyl (3h and 3j). The size of either the groups connected to the carbonyl or the ester groups determines the outcomes of the reactions. For example, with the increase of the size of ester groups, the reactivity and stereoselectivity of the nucleophiles both decreased (3n–p). Although a smaller cyclopropyl substituted ethyl acetoacetate gave poor diastereoselectivity and moderate enantioselectivity (3q), larger cyclopentyl and cyclohexyl substituents afforded the target products in good yields and excellent stereoselectivities (3r and 3s). Heterocyclic substituents are compatible with the reaction (3t and 3u).
1 dr, up to >99% ee). In contrast, steric factor plays an important role in the reactivity and stereoselectivity of the substrates. For instance, m-bromo substituted substrate (3l) required a higher reaction temperature (ambient temperature)9 to afford the product but with a poorer dr and ee than those bearing smaller substituents such as methyl (3j) or chlorine atom (3k). O-Methyl (3m) also required a higher reaction temperature than m- and p-methyl (3h and 3j). The size of either the groups connected to the carbonyl or the ester groups determines the outcomes of the reactions. For example, with the increase of the size of ester groups, the reactivity and stereoselectivity of the nucleophiles both decreased (3n–p). Although a smaller cyclopropyl substituted ethyl acetoacetate gave poor diastereoselectivity and moderate enantioselectivity (3q), larger cyclopentyl and cyclohexyl substituents afforded the target products in good yields and excellent stereoselectivities (3r and 3s). Heterocyclic substituents are compatible with the reaction (3t and 3u).
More importantly, a diverse variety of 3° C–H nucleophiles exhibited equal reactivities and stereoselectivities. Substrates that differed in aromatic, aliphatic or acyclic carbon skeletons, both esters and lactones, all constructed the desired all-carbon quaternary stereocenters in high efficiencies (3v–z). Interestingly, the existence of an ester subunit in the substrates was proven to be vital to both reactivity and stereoselectivity. For example, 1,3-diketones, both 2° and 3° C–H nucleophiles (3aa and 3ab), gave less yields and dramatic drops on the stereoselectivities in contrast to β-ketoesters. The different coordination properties between esters and carbonyls may account for the phenomenon.
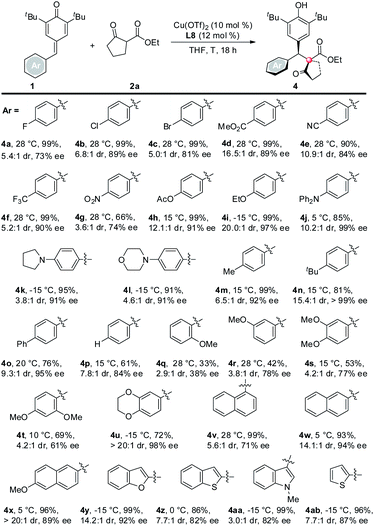
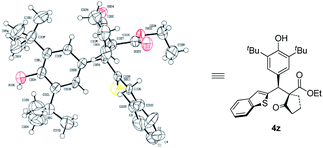
Next, the scope of p-QMs was investigated (Table 3). The data revealed that both the electronic and steric factors are crucial to the reactivity and stereoselectivity of the p-QMs. In general, p-QMs bearing electron-poor aryls are less reactive towards the nucleophiles. They required an elevated temperature in order to provide the target products. However, most of them still maintained good yields and stereoselectivities (4a–h). In contrast, other substrates bearing electron-rich aryls have comparable reactivity with 1a. They reacted with the nucleophiles at lower temperatures with good yields and stereoselectivities (4i–o). For a p-QM bearing an electron-neutral phenyl, interestingly, both the yield and stereoselectivity were moderate (4p), which indicated that electronic factor is not the only element that determines the outcome of the reaction. And once more, the steric factor played a crucial role in the reaction. For example, more sterically hindered o- and m-methoxy substituted substrates required higher reaction temperatures while gave modest yields and even poorer ee's (4q and 4r). p-QMs bearing di-substituted aryls afforded the target product with moderate to good stereoselectivities (4s–u), which was attributed to the increased steric hindrance. Fused rings containing substrates also proceeded well (4v–x). As anticipated, more steric hindered 1-naphthyl (4v) required a higher reaction temperature but afforded lower dr and ee than its 2-naphthyl counterpart (4w). Again, heterocyclic rings are compatible with the reaction (4y–ab).10 The relative and absolute configuration of 4z was unequivocally determined by single crystal X-ray diffraction (Scheme 2).11
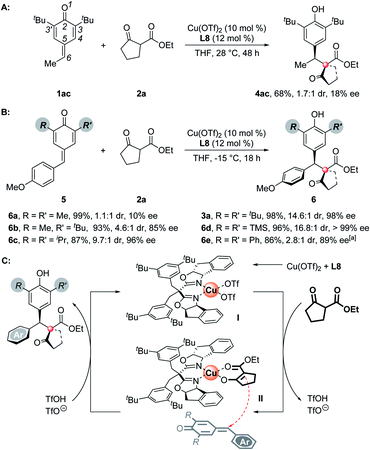
To understand the substituents' influence on the reactivity and stereoselectivity of the p-QMs, a series of substrates with different substituents on the p-QM skeletons were tested. First, the aromatic rings on the C-6 position of the p-QMs are necessary to maintain a high level of stereoselectivity, for a C-6 methyl substituted substrate suffered both low reactivity and poor stereoselectivity (Scheme 3A). Besides, the substituents on the C-3 position are important as well. For methyl, the smallest substituent tested, it brings a poor discrimination between the Si- and Re-faces of the p-QM planes, thus resulting in the formation of unselective products (Scheme 3B, 6a). When a 3-methyl-3'-tert-butyl substrate was employed, the stereoselectivity improved but was still moderate (6b). Isopropyl, which has a smaller size than tert-butyl, afforded the product slightly less stereoselectively than the tert-butyl did (6c). Interestingly, a bigger TMS group exhibited modestly better steric control (6d). Unlike alkyl substituents, C-3 aryl rings substituents don't obey the above-mentioned “size law”, showing a deleterious effect on the reactivity and stereoselectivity (6e). On the basis of all of the experimental results, the high stereoselectivity of this reaction may owe to the combined interaction among the chiral catalyst, p-QMs, and the nucleophiles. Thus, a possible catalytic cycle and a plausible transition state which determines the absolute configuration of the products are proposed (Scheme 3C). Once the chiral Lewis acid catalyst I was generated from Cu(OTf)2 and the box ligand, the subsequent ligand exchange and deprotonation of 1,3-dicarbonyl nucleophiles formed an intermediate II, which then underwent an asymmetric 1,6-addition with the p-QMs to afford the final chiral product with high levels of entioselectivity and diastereoselectivity. The presumed transition state rationalizes the observed absolute stereochemical outcome. In this model, the chiral catalyst determines a Si-face attack on the p-QM planes, the ester groups of nucleophiles are opposite the aryls of p-QMs to minimize the steric hindrance or provide additional π-stacking interactions (for 3b–m, 3x).
 | ||
| Scheme 3 Substituents' influence on stereoselectivity and proposed mechanism. aReaction conducted at −5 °C. | ||
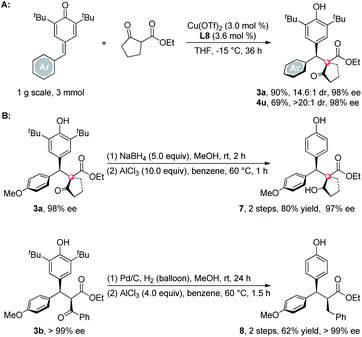
The reaction can be carried out in gram-scale using 3 mol% catalyst loading without the destruction on yields and ee's, only at the expense of prolonged reaction times (Scheme 4A). Although these synthesized products are unstable under the acidic AlCl3-induced de-tert-butylation process,12 after reducing the carbonyls to either hydroxyls (3a) or methylene groups (3b),13 the tert-butyls on the aryl rings can be readily removed by AlCl3 without any loss of the optical purities (Scheme 4B).
Conclusions
In conclusion, we have developed a simple but efficient protocol to construct two contiguous stereocenters including one all-carbon quaternary stereocenter from a broad scope of p-QMs and 1,3-dicarbonyl compounds with high atom economy. Good yields and excellent stereoselectivities are achieved in most cases. The application of the copper-chiral bisoxazoline catalytic system to other asymmetric synthesis is under investigated in our group.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the finance support from the National Natural Science Foundation (21772046 and 2193103).Notes and references
- For reviews, see: (a) T. C. Nugent, Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH, Weinheim, Germany, 2010 CrossRef; (b) F. Schmidt, R. T. Stemmler, R. Rudolph and C. Bolm, Chem. Soc. Rev., 2006, 35, 454–470 CAS; (c) D. Ameen and T. J. Snape, MedChemComm, 2013, 4, 893–907 RSC.
- For selected reviews on quinone methide, see: (a) Quinone Methides, ed, S. E. Rokita, Wiley, Hoboken, 2009 Search PubMed; (b) T. P. Pathak and M. S. Sigman, J. Org. Chem., 2011, 76, 9210–9215 CrossRef CAS PubMed; (c) N. J. Willis and C. D. Bray, Chem.–Eur. J., 2012, 18, 9160–9173 CrossRef CAS PubMed; (d) Z. Wang and J. Sun, Synthesis, 2015, 47, 3629–3644 CrossRef; (e) L. Caruana, M. Fochi and L. Bernardi, Molecules, 2015, 20, 11733–11764 CrossRef CAS PubMed; (f) A. Parra and M. Tortosa, ChemCatChem, 2015, 7, 1524–1526 CrossRef CAS; (g) W. Li, X. Xu, P. Zhang and P. Li, Chem.–Asian J., 2018, 13, 2350–2359 CrossRef CAS PubMed.
- (a) W.-D. Chu, L.-F. Zhang, X. Bao, X.-H. Zhao, C. Zeng, J.-Y. Du, G.-B. Zhang, F.-X. Wang, X.-Y. Ma and C.-A. Fan, Angew. Chem., Int. Ed., 2013, 52, 9229–9233 CrossRef CAS PubMed; (b) L. Caruana, F. Kniep, T. K. Johansen, P. H. Poulsen and K. A. Jørgensen, J. Am. Chem. Soc., 2014, 136, 15929–15932 CrossRef CAS PubMed; (c) K. Zhao, Y. Zhi, T. Shu, A. Valkonen, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 12104–12108 CrossRef CAS PubMed; (d) S. Li, Y. Liu, B. Huang, T. Zhou, H. Tao, Y. Xiao, Y. Liu and J. Zhang, ACS Catal., 2017, 7, 2805–2809 CrossRef CAS; (e) F.-S. He, J.-H. Jin, Z.-T. Yang, X. Yu, J. S. Fossey and W.-P. Deng, ACS Catal., 2016, 6, 652–656 CrossRef CAS; (f) L. Zhang, H. Yuan, W. Lin, Y. Cheng, P. Li and W. Li, Org. Lett., 2018, 20, 4970–4974 CrossRef CAS PubMed; (g) Z. Sun, B. Sun, N. Kumagai and M. Shibasaki, Org. Lett., 2018, 20, 3070–3073 CrossRef CAS PubMed; (h) X. Li, X. Xu, W. Wei, A. Lin and H. Yao, Org. Lett., 2016, 18, 428–431 CrossRef CAS PubMed; (i) X.-Z. Zhang, K.-J. Gan, X.-X. Liu, Y.-H. Deng, F.-X. Wang, K.-J. Yu, J. Zhang and C.-A. Fan, Org. Lett., 2017, 19, 3207–3210 CrossRef CAS PubMed; (j) X.-Z. Zhang, Y.-H. Deng, X. Yan, K.-Y. Yu, F.-X. Wang, X.-Y. Ma and C.-A. Fan, J. Org. Chem., 2016, 81, 5655–5662 CrossRef CAS PubMed; (k) L. Ge, X. Lu, C. Cheng, J. Chen, W. Cao, X. Wu and G. Zhao, J. Org. Chem., 2016, 81, 9315–9325 CrossRef CAS PubMed; (l) T.-C. Kang, L.-P. Wu, Q.-W. Yu and X.-Y. Wu, Chem.–Eur. J., 2017, 23, 6509–6513 CrossRef CAS PubMed; (m) Q. Liu, S. Li, X.-Y. Chen, K. Rissanen and D. Enders, Org. Lett., 2018, 20, 3622–3626 CrossRef CAS PubMed; (n) W. Li, X. Xu, Y. Liu, H. Gao, Y. Cheng and P. Li, Org. Lett., 2018, 20, 1142–1145 CrossRef CAS PubMed; (o) K. J. Romero, M. S. Galliher, M. A. R. Raycroft, J.-P. R. Chauvin, I. Bosque, D. A. Pratt and C. R. J. Stephenson, Angew. Chem., Int. Ed., 2018, 57, 17125–17129 CrossRef CAS PubMed; (p) G.-B. Huang, W.-H. Huang, J. Guo, D.-L. Xu, X.-C. Qu, P.-H. Zhai, X.-H. Zheng, J. Weng and G. Lu, Adv. Synth. Catal., 2019, 361, 1241–1246 CrossRef CAS; (q) Y. Wang, K. Wang, W. Cao, X. Liu and X. Feng, Org. Lett., 2019, 21, 6063–6067 CrossRef CAS PubMed; (r) D. Wang, Z.-F. Song, W.-J. Wang and T. Xu, Org. Lett., 2019, 21, 3963–3967 CrossRef CAS PubMed; (s) J.-R. Wang, X.-L. Jiang, Q.-Q. Hang, S. Zhang, G.-J. Mei and F. Shi, J. Org. Chem., 2019, 84, 7829–7839 CrossRef CAS PubMed.
- For C–B bond formation, see: (a) Y. Lou, P. Cao, T. Jia, Y. Zhang, M. Wang and J. Liao, Angew. Chem., Int. Ed., 2015, 54, 12134–12138 CrossRef CAS PubMed; (b) C. Jarava-Barrera, A. Parra, A. López, F. Cruz-Acosta, D. Collado-Sanz, D. J. Cárdenas and M. Tortosa, ACS Catal., 2016, 6, 442–446 CrossRef CAS PubMed; For C–S bond formation, see: (c) N. Dong, Z.-P. Zhang, X.-S. Xue, X. Li and J.-P. Cheng, Angew. Chem., Int. Ed., 2016, 55, 1460–1464 CrossRef CAS PubMed; For C–N bond formation, see: (d) F. Jiang, F.-R. Yuan, L.-W. Jin, G.-J. Mei and F. Shi, ACS Catal., 2018, 8, 10234–10240 CrossRef CAS.
- For selected reviews on all-carbon quaternary stereocenter, see: (a) E. J. Corey and A. Guzman-Perez, Angew. Chem., Int. Ed., 1998, 37, 388–401 CrossRef; (b) K. W. Quasdorf and L. E. Overman, Nature, 2014, 516, 181–191 CrossRef CAS PubMed; (c) M. Büschleb, S. Dorich, S. Hanessian, D. Tao, K. B. Schenthal and L. E. Overman, Angew. Chem., Int. Ed., 2016, 55, 4156–4186 CrossRef PubMed; (d) B. M. Trost and C. Jiang, Synthesis, 2006, 369–396 CrossRef CAS; (e) Y. Liu, S.-J. Han, W.-B. Liu and B. M. Stoltz, Acc. Chem. Res., 2015, 48, 740–751 CrossRef CAS PubMed; (f) J. Feng, M. Holmes and M. J. Krische, Chem. Rev., 2017, 117, 12564–12580 CrossRef CAS PubMed; (g) B. Wang and Y.-Q. Tu, Acc. Chem. Res., 2011, 44, 1207–1222 CrossRef CAS PubMed; (h) R. Long, J. Huang, J. Gong and Z. Yang, Nat. Prod. Rep., 2015, 32, 1584–1601 RSC; (i) P. W. Xu, J.-S. Yu, C. Chen, Z.-Y. Cao, F. Zhou and J. Zhou, ACS Catal., 2019, 9, 1820–1882 CrossRef CAS; (j) X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou and J. Zhou, Chem. Rev., 2016, 116, 7330–7396 CrossRef CAS PubMed; (k) L. M. Repka and S. E. Reisman, J. Org. Chem., 2013, 78, 12314–12320 CrossRef CAS PubMed.
- (a) K. Zhao, Y. Zhi, A. Wang and D. Enders, ACS Catal., 2016, 6, 657–660 CrossRef CAS; (b) Y.-H. Deng, X.-Z. Zhang, K.-Y. Yu, X. Yan, J.-Y. Du, H. Huang and C.-A. Fan, Chem. Commun., 2016, 52, 4183–4186 RSC.
- See ESI† for more details.
- A dramatically low conversion was obtained when the reaction conducted at −20 °C. See ESI† for details.
- During this work, the average ambient temperature in our lab was 28 °C..
- p–QM substrates bearing strong coordinated quinolone and pyridine rings gave no conversion under the standard reaction conditions.
- See ESI† for more details.
- These products are very sensitive toward either strong acidic or basic conditions, and decompose into the two starting materials very quickly.
- S. A. Saleh and H. I. Tashtoush, Tetrahedron, 1998, 54, 14157–14177 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1958259, 1963665 and 1963687. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc00142b |
| This journal is © The Royal Society of Chemistry 2020 |